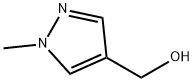
(1-Methyl-1H-pyrazol-4-yl)methanol synthesis
- Product Name:(1-Methyl-1H-pyrazol-4-yl)methanol
- CAS Number:112029-98-8
- Molecular formula:C5H8N2O
- Molecular Weight:112.13
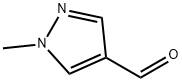
25016-11-9

112029-98-8
General procedure for the synthesis of 1-methyl-4-(hydroxymethyl)pyrazole from 1-methyl-1H-pyrazole-4-carboxaldehyde: 1-methyl-1H-pyrazole-4-carboxaldehyde (3.48 g, 31.6 mmol) was dissolved in methanol (25 mL), and sodium borohydride (2.50 g, 66.1 mmol, 2.09 eq.) was added in batches under conditions of vigorous gas release. The reaction mixture was stirred at room temperature for 3 h. The reaction mixture was then cooled to 0 °C and acidified by slow addition of aqueous 5N hydrochloric acid (20 mL) over 55 min to a pH of about 1. A thick white slurry was formed during the reaction and stirring was continued for 1 h at room temperature. Subsequently, the reaction mixture was alkalized by slow addition of saturated aqueous potassium carbonate solution (53.4 wt% K2CO3, 6.04 M; 10 mL). A clarified colorless solution (pH = 1.1) was obtained, which was diluted with additional saturated potassium carbonate solution (200 mL) and extracted with ethyl acetate (2 × 200 mL). The ethyl acetate extracts were combined, dried over anhydrous sodium sulfate, filtered and concentrated to give 1-methyl-4-(hydroxymethyl)pyrazole as a light yellow oil (3.44 g, 97% yield).

25016-11-9
242 suppliers
$5.00/1g

112029-98-8
111 suppliers
$18.00/1g
Yield: 97%
Reaction Conditions:
Stage #1:1-methylpyrazole-4-carbaldehyde with sodium tetrahydroborate in methanol at 20; for 3 h;
Stage #2: with hydrogenchloride;methanol in water; pH=1 at 0 - 20;
Steps:
3
The compound of formula IX (3.48 g, 31.6 mmol) was dissolved in methanol (25 ml_) and sodium borohydride (2.50 g, 66.1 mmol, 2.09 equiv.) was added portion-wise with vigorous gas evolution. After stirring for 3 hours at room temperature, the reaction was cooled to 0 0C and slowly acidified to pH ~ 1 with 4N aqueous hydrochloric acid (20 ml_) over 55 minutes. A thick white slurry formed and this was stirred one hour at room temperature. The reaction was then basified by the gradual addition of saturated aqueous potassium carbonate solution (53.4 wt% K2CU3, 6.04 M; 10 ml_). This resulted in a clear, colorless solution (pH = 1.1), which was diluted with additional saturated potassium carbonate solution (200 ml_) and was extracted with ethyl acetate (2 x 200 ml_). The ethyl acetate extracts were combined, dried over sodium sulfate, filtered and concentrated to yield the compound of formula X as a light yellow oil (3.44 g, 97% yield).
References:
SCHERING CORPORATION WO2008/153870, 2008, A1 Location in patent:Page/Page column 30
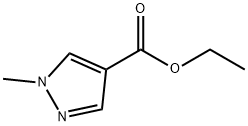
85290-80-8
99 suppliers
$44.00/250 mg

112029-98-8
111 suppliers
$18.00/1g
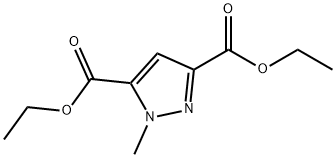
100852-80-0
64 suppliers
$57.50/250 mg

112029-98-8
111 suppliers
$18.00/1g