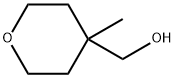
4-HydroxyMethyl-4-Methyltetrahydro-2H-pyran synthesis
- Product Name:4-HydroxyMethyl-4-Methyltetrahydro-2H-pyran
- CAS Number:502609-47-4
- Molecular formula:C7H14O2
- Molecular Weight:130.18
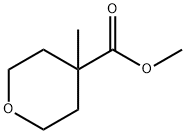
443912-70-7
65 suppliers
$50.00/250mg

502609-47-4
53 suppliers
$45.00/25mg
Yield:502609-47-4 100%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether at 0 - 20; for 4 h;
Steps:
7.1 PREPARATION EXAMPLE 7; Preparation of 3-(4-methyltetrahydropyran-4-yl)propenal (VII-143)
1) To a cooled (0°C) solution of 3.45 g (21.8 mmol) of 4-methyltetrahydropyran-4-carboxylic acid methyl ester (IX-60) obtained in PREPARATION EXAMPLE 1 in ether (70 ML) was added 830 mg (21.8 mmol) of lithium aluminum hydride under an atmosphere of argon, and stirred for 1 hour at 0°C, and then for another 1 hour at room temperature.. After the reaction mixture was cooled again to 0°C, 830 mg (21.8 mmol) of lithium aluminum hydride was added and stirred for 1 hour at 0°C, and subsequently for 1 hour at room temperature.. The reaction mixture was diluted with ether, and then excess reagent was carefully quenched with ethanol (8 ML) and saturated brine (10ML) at 0°C. Upon drying the reaction mixture with anhydrous magnesium sulfate and subsequent evaporation of the solvent, 3.64 g (yield 100 %) of (4-methylteterahydropyran-4-yl)methanol was obtained as colorless liquid.. Its physical property is shown below.1H-NMR(CDCl3) δ value: 1.04(3H, s), 1.29(2H, m), 1.59(3H, m), 3.40(2H, d, J=5.6), 3.54-3.82(4H, m).
References:
EP1431285,2004,A1 Location in patent:Page 107-108
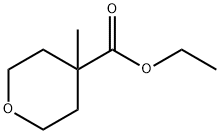
861161-92-4
3 suppliers
inquiry

502609-47-4
53 suppliers
$45.00/25mg
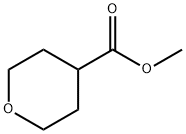
110238-91-0
213 suppliers
$13.00/5g

502609-47-4
53 suppliers
$45.00/25mg
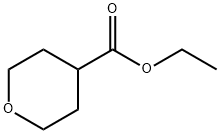
96835-17-5
84 suppliers
$19.00/1g

502609-47-4
53 suppliers
$45.00/25mg