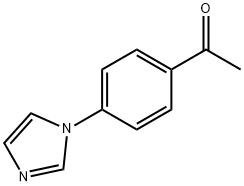
4'-(IMIDAZOL-1-YL)ACETOPHENONE synthesis
- Product Name:4'-(IMIDAZOL-1-YL)ACETOPHENONE
- CAS Number:10041-06-2
- Molecular formula:C11H10N2O
- Molecular Weight:186.21
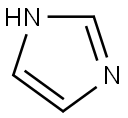
288-32-4
1039 suppliers
$5.00/25g
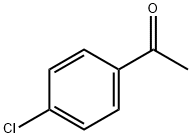
99-91-2
434 suppliers
$10.00/25g

10041-06-2
130 suppliers
$36.00/1g
Yield:10041-06-2 92%
Reaction Conditions:
with copper(l) iodide;potassium carbonate;1-(2-pyridyl)-3-(2-pyridyl)-1,3-propanedione in N,N-dimethyl-formamide at 20 - 110; for 18.5 h;Inert atmosphere;
Steps:
1-[4-(1H-Imidazol-1-yl)phenyl]ethanone (3)
General procedure: The compound was prepared according to a modified literature procedure.22 A mixture of imidazole (1.021 g, 15 mmol), anhydrous potassium carbonate (2.76 g, 20 mmol), copper iodide (0.119 g,1.0 mmol), 1,3-di(pyridin-2-yl)propane-1,3-dione (10.0 mmol), 4-haloacetophenone (10.0 mmol) and DMF (15 mL) was stirred for 30 min at room temperature under a nitrogen atmosphere. This wa sfollowed by heating at 110 °C for 12-18 h. After the completion of the reaction (as indicated by TLC), the contents were cooled to room temperature and passed through a plug of Celite. The filtrate was diluted with ethyl acetate (50 mL) and washed three times with saturated brine (10 mL each time). Treatment of the organic layer with activated charcoal and evaporation of the solvent under vacuum afforded the product: Yield (see Table 1); m.p. 115-116 °C (lit.22 114-116 °C).
References:
Hussain, Tanvir;Zia-Ur-Rehman, Muhammad;Zaheer, Muhammad;Ashraf, Chouhdary Muhammad;Bolte, Michael [Journal of Chemical Research,2016,vol. 40,# 4,p. 199 - 204]

288-32-4
1039 suppliers
$5.00/25g
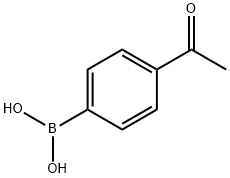
149104-90-5
291 suppliers
$10.00/250mg

10041-06-2
130 suppliers
$36.00/1g

288-32-4
1039 suppliers
$5.00/25g
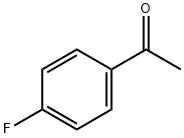
403-42-9
553 suppliers
$5.00/10g

10041-06-2
130 suppliers
$36.00/1g

288-32-4
1039 suppliers
$5.00/25g
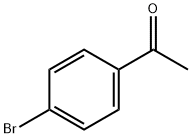
99-90-1
476 suppliers
$6.00/25g

10041-06-2
130 suppliers
$36.00/1g

288-32-4
1039 suppliers
$5.00/25g
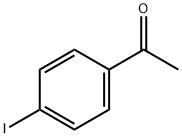
13329-40-3
273 suppliers
$6.00/5g

10041-06-2
130 suppliers
$36.00/1g