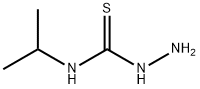
4-ISOPROPYL-3-THIOSEMICARBAZIDE synthesis
- Product Name:4-ISOPROPYL-3-THIOSEMICARBAZIDE
- CAS Number:13431-36-2
- Molecular formula:C4H11N3S
- Molecular Weight:133.22
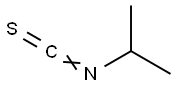
2253-73-8
210 suppliers
$10.00/5g

13431-36-2
72 suppliers
$36.25/1g
Yield: 95.8%
Reaction Conditions:
with hydrazine in ethanol at 20; for 0.5 h;
Steps:
2.13.1
The First Step: Synthesis of F63-01 While stirring in ice cold conditions, isopropylisocyanate (2-isothiocyanato-propane) (3.0 mL, 28.2 mmol) was added to an ethanol (10 mL) solution of hydrazine monohydrate (2.88 g, 56.4 mmol). After stirring at room temperature for 30 minutes, the solvent was distilled off under reduced pressure. The residue was extracted with chloroform, and the extract was washed twice with water and then with saturated sodium chloride solution. After drying over anhydrous sodium, sodium sulfate was removed by filtration and the solvent was distilled off under reduced pressure. Solids thus obtained were dried under reduced pressure to obtain F-63-01 (white solids, 3.59 g, 95.8%). LC/MS (Method 3): m/z (ESI, POS) : 134[M+H]+; retention time: 2.19 minutes. 1H-NMR(400MHz, CDCl3)? 1.26(d, J=6.6Hz, 6H), 3.71(s, 2H), 4.49-4.58(m, 1H), 7.14(bs, 1H), 7.25(br, 1H).
References:
NIPPON KAYAKU KABUSHIKI KAISHA EP1857446, 2007, A1 Location in patent:Page/Page column 55
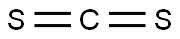
75-15-0
272 suppliers
$40.00/100 g
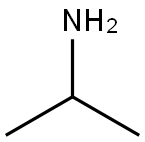
75-31-0
443 suppliers
$14.00/25mL

13431-36-2
72 suppliers
$36.25/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

13431-36-2
72 suppliers
$36.25/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

13431-36-2
72 suppliers
$36.25/1g