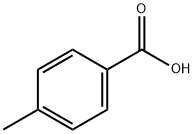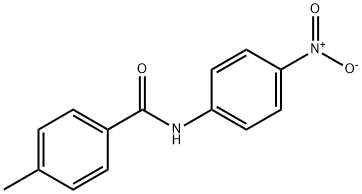
4-Methyl-N-(4-nitrophenyl)benzaMide synthesis
- Product Name:4-Methyl-N-(4-nitrophenyl)benzaMide
- CAS Number:33667-88-8
- Molecular formula:C14H12N2O3
- Molecular Weight:256.26
874-60-2
413 suppliers
$10.00/5g
100-01-6
72 suppliers
$11.19/25G

33667-88-8
5 suppliers
$28.60/50MG
Yield:33667-88-8 92%
Reaction Conditions:
in neat (no solvent); for 0.0583333 h;Microwave irradiation;Green chemistry;
Steps:
General procedure for the synthesis of N-(aryl)- substituted benzamides [IIIa to IIIt]
General procedure: The reactants aniline, 250mg (2 mmol) and acid chloride, 250 mg (2 mmol) were thoroughly mixed in a crucible and exposure to microwave irradiation under solvent-free conditions using modified domestic microwave at 495 W for 3.5 min (Table I). The reaction course was carefully monitored by thin layer chromatogram (TLC) to regulate the reactant ratio, irradiation time, and microwave power level to achieve the maximum yield. The crude product (a powder), N-aryl- substituted benzamides thus obtained was washed with water and purified by recrystallization using 95% alcohol and dried under vacuum (Table I).
References:
Rao, Sri Latha;Veerabhadraswamy;Molkere, Bhimashankar B. [Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry,2020,vol. 59,# 6,p. 850 - 855]
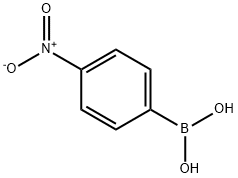
24067-17-2
259 suppliers
$19.00/1g
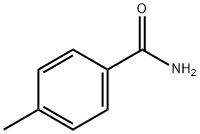
619-55-6
199 suppliers
$5.00/1g

33667-88-8
5 suppliers
$28.60/50MG
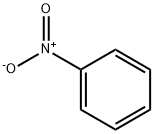
98-95-3
34 suppliers
$14.00/25g

619-55-6
199 suppliers
$5.00/1g

33667-88-8
5 suppliers
$28.60/50MG
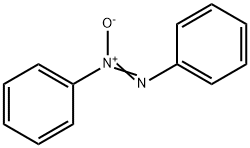
495-48-7
90 suppliers
$39.59/10g