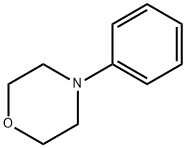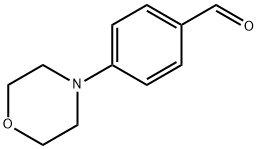
4-Morpholinobenzaldehyde synthesis
- Product Name:4-Morpholinobenzaldehyde
- CAS Number:1204-86-0
- Molecular formula:C11H13NO2
- Molecular Weight:191.23

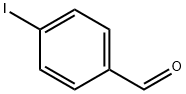
110-91-8
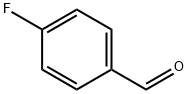
459-57-4

1204-86-0
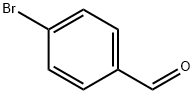
General procedure for the synthesis of 4-(4-morpholine)benzaldehyde from morpholine and p-fluorobenzaldehyde: In a dry reaction flask, p-fluorobenzaldehyde (25.0 g, 0.200 mol), morpholine (0.300 mol), and anhydrous potassium carbonate (40.0 g) were dissolved in N,N-dimethylformamide (DMF, 300 mL), and a catalytic amount of Aliquat 336 reagent. The reaction mixture was stirred at reflux for 24 hours at 100°C. Upon completion of the reaction, the mixture was concentrated to dryness under reduced pressure and cooled to room temperature. Subsequently, the concentrate was slowly poured into ice water and allowed to stand overnight. The precipitated solid was collected by filtration, washed with water and recrystallized with methanol to afford the target compound 4-morpholino benzaldehyde (Id) in 89% yield as yellow crystals with a melting point of 69 °C (in agreement with literature reports) [49,50].
Yield:1204-86-0 86 %Chromat.
Reaction Conditions:
with C35H34N3OP2PdS(1+)*NO3(1-);sodium t-butanolate in 1,4-dioxane at 100; for 6 h;Catalytic behavior;Buchwald-Hartwig Coupling;
Steps:
2.5.2. General procedure for Buchwald-type C-N coupling reactions
General procedure: In a typical run, an oven-dried 10 ml round bottom flask was charged with a known mole percent of catalyst, NaOtBu (1.3 mmol), amine (1.2 mmol) and aryl halide (1 mmol) with the appropriate solvent(s) (4 ml). The flask was placed in a preheated oil bath at required temp. After the specified time the flask was removed from the oil bath, water (20 ml) was added, and extraction with ether (4×10 ml) was done. The combined organic layers were washed with water (3×10 ml), dried over anhydrous Na2SO4, and filtered. Solvent was removed under vacuum. The residue was dissolved in acetonitrile and analyzed by GC-MS.
References:
Thapa, Kiran;Paul, Piyali;Bhattacharya, Samaresh [Inorganica Chimica Acta,2019,vol. 486,p. 232 - 239]
![Morpholine, 4-[4-(1,3-dioxolan-2-yl)phenyl]-](/CAS/20210305/GIF/773097-39-5.gif)
773097-39-5
1 suppliers
inquiry

1204-86-0
164 suppliers
$8.00/1g