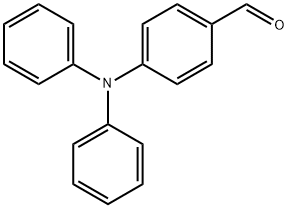
4-(N,N-Diphenylamino)benzaldehyde synthesis
- Product Name:4-(N,N-Diphenylamino)benzaldehyde
- CAS Number:4181-05-9
- Molecular formula:C19H15NO
- Molecular Weight:273.33
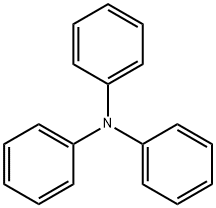
603-34-9

4181-05-9
Phosphoryl chloride (24 mL, 315 mmol) was slowly added dropwise to N,N-dimethylformamide (DMF, 30 mL) at 0 °C and the reaction mixture was stirred for 1 h keeping this temperature. Subsequently, triphenylamine (10 g, 40 mmol) was added and the reaction system was warmed to 100 °C with continuous stirring for 6 hours. After completion of the reaction, the mixture was cooled to room temperature, slowly poured into ice water and the pH was adjusted with 5% aqueous sodium hydroxide to 7. The aqueous phase was extracted with ethyl acetate and the combined organic phases were dried with anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure to give the crude product. The crude product was purified by column chromatography using a mixed solvent of petroleum ether and dichloromethane (2:1, v/v/v) as eluent, and finally 4-diphenylaminobenzaldehyde was obtained as a white solid (5.76 g) in 52.74% yield.

75-09-2
1263 suppliers
$10.00/25 mL

603-34-9
315 suppliers
$24.89/5g

67-64-1
0 suppliers
$17.30/10ml

4181-05-9
209 suppliers
$14.00/1g
Yield:4181-05-9 73%
Reaction Conditions:
with sodium acetate trihydrate;magnesium sulfate;trichlorophosphate in methanol
Steps:
1.1 Synthesis of 4-(diphenylamino)benzaldehyde
EXAMPLE 1-1 Synthesis of 4-(diphenylamino)benzaldehyde 30 mL of dimethylformaldehyde (DMF) was put into a 250 mL flask, cooled to 0° C. and 3.8 mL of POCl3 was slowly added thereto. Then, 10 g of triphenylamine was added to the resultant mixture and heated to 70° C. for 5 hours. The reaction container was cooled to room temperature and then a reactant solution was poured into ice water. Subsequently, 40 g of sodium acetate trihydrate was put for neutralization and then an organic layer was separated with methylenechloride. The obtained organic layer was dried using magnesium sulfate and methylenechloride was removed under reduced pressure, yielding a product. The product was recrystallized using methanol and a light yellow solid was finally obtained, yielding 73% of a desired product. Data of NMR spectra confirmed that the product had the same structure as 4-(diphenylamino)benzaldehyde, that is, the compound (1) in the reaction scheme 2.
References:
Lee, Jeong Ik;Chu, Hye Yong;Lee, Hyoyoung;Oh, Ji Young;Kim, Seong Hyun;Do, Lee-Mi;Zyung, Taehyoung;Lee, Jaemin;Jung, Byung-Jun;Shim, Hong-Ku US2003/87127, 2003, A1

603-34-9
315 suppliers
$24.89/5g

4181-05-9
209 suppliers
$14.00/1g
20441-00-3
25 suppliers
inquiry

4181-05-9
209 suppliers
$14.00/1g
1122-91-4
702 suppliers
$9.00/10g
122-39-4
357 suppliers
$14.00/5g

4181-05-9
209 suppliers
$14.00/1g
![Benzenamine, N,N-diphenyl-4-[2-(triethoxysilyl)ethyl]-](/CAS/20210305/GIF/190334-72-6.gif)