
4-N-PROPYLTHIOPHENOL synthesis
- Product Name:4-N-PROPYLTHIOPHENOL
- CAS Number:4527-44-0
- Molecular formula:C9H12S
- Molecular Weight:152.26
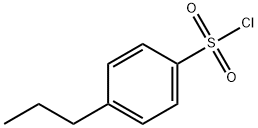
146949-07-7
80 suppliers
$21.00/1g

4527-44-0
13 suppliers
$809.00/1g
Yield:4527-44-0 72%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether at 35; for 24 h;Inert atmosphere;
Steps:
General procedure for the synthesis of arylthiols 12a-e
General procedure: To a solution of sulfonyl chloride derivative 11 (9.85 mmol)in anhydrous diethyl ether (15 mL) under an atmosphere ofnitrogen was added LiAlH4 (0.560 g, 14.8 mmol) in portions.The reaction mixture was then refluxed at 35 °C for24 h upon which TLC analysis showed reaction completion.The reaction was then cooled on ice and quenched by slowaddition of a 10% solution of aqueous Na2SO4. The solidswere then filtered off and washed several times with hotethyl acetate. The filtrate was then concentrated underreduced pressure and the crude products thus isolated werepurified by silica gel column chromatography using hexaneas the eluent to yield the corresponding thiophenols 12. 4-Propylbenzenethiol (12a): colorless oil, 72% (1.50 g),IR (neat cm-1): 2973, 2900, 1608, 1504, 1460, 1154, 1085;1H NMR (400 MHz, CDCl3) δ: 7.19 (d, J = 8.0 Hz, 2H,Ar), 7.04 (d, J = 8.0 Hz, 2H, Ar), 3.38 (s, 1H, SH), 2.52 (t,J = 7.6 Hz, 2H, H-1), 1.74-1.55 (m, 2H, H-2), 0.91 (t, J = 7.4 Hz, 3H, H-3); 13C NMR (100MHz, CDCl3) δ: 140.5,129.8, 129.2, 126.8 (Ar), 37.4 (C-1), 24.5 (C-2), 13.7 (C-3).HRMS (ESI): m/z [M - H+] Calcd for C9H11S: 151.0581;found 151.0581.
References:
Madumo, Gilbert K.;Moshapo, Paseka T.;Kinfe, Henok H. [Medicinal Chemistry Research,2018,vol. 27,# 3,p. 817 - 833]
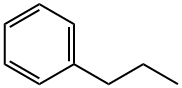
103-65-1
173 suppliers
$18.00/25mL

4527-44-0
13 suppliers
$809.00/1g
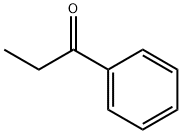
93-55-0
0 suppliers
$15.00/25g

4527-44-0
13 suppliers
$809.00/1g
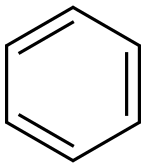
71-43-2
721 suppliers
$11.19/25ML

4527-44-0
13 suppliers
$809.00/1g