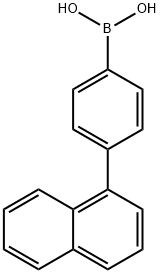
4-(NAPHTHALEN-1-YL)PHENYLBORONIC ACID synthesis
- Product Name:4-(NAPHTHALEN-1-YL)PHENYLBORONIC ACID
- CAS Number:870774-25-7
- Molecular formula:C16H13BO2
- Molecular Weight:248.08
204530-94-9

870774-25-7
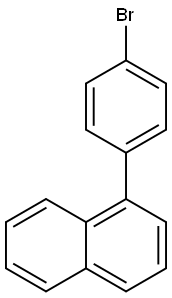
A mixed solution of 208.8 g (737.4 mmol) of 1-(4-bromophenyl)naphthalene with 2.1 L of anhydrous tetrahydrofuran (THF) was cooled to -60 °C under argon protection. Subsequently, 567 mL (884.9 mmol) of 1.56 M n-butyllithium hexane solution was added slowly dropwise under stirring conditions. After the dropwise addition was completed, the reaction mixture was continued to be stirred at -60 °C for 2 hours. Maintaining the temperature at -60 °C, 416 g (2.21 mol) of triisopropyl borate was added dropwise to the reaction system. After completion of the dropwise addition, the reaction mixture was gradually warmed to room temperature and stirred for 17 hours. At the end of the reaction, an aqueous hydrochloric acid solution was added to the mixture and stirred for 1 hour at room temperature. The reaction mixture was extracted with toluene, and the aqueous phase was separated and discarded. The organic phase was dried with magnesium sulfate and the solvent was removed by distillation under reduced pressure. The resulting solid was purified by recrystallization from toluene to give 126 g of 4-(1-naphthyl)phenylboronic acid in 67% yield.

204530-94-9
199 suppliers
$6.00/250mg

870774-25-7
226 suppliers
$6.00/1g
Yield:870774-25-7 67%
Reaction Conditions:
Stage #1: 1-(4-bromo-phenyl)-naphthalenewith n-butyllithium in tetrahydrofuran;hexane at -60; for 2 h;Inert atmosphere;
Stage #2: with Triisopropyl borate in tetrahydrofuran;hexane at -60 - 20; for 17 h;Inert atmosphere;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;water at 20; for 1 h;
Steps:
4.2 [Synthesis Reference 4-2] Synthesis of 4-(1-naphthyl)phenylboronic acid
Under an argon gas atmosphere, a mixture of 208.8 g (737.4 mmol) of 1-(4-bromophenyl)naphthalene and 2.1 L of dehydrated TI-IF was cooled down to minus 60 degree C. Then, 567 ml (884.9 mmol) of hexane solution of 1.56M n-butyllithium was dropped into the mixture while the mixture was being stirred. The reaction mixture was further stirred at minus 60 degrees for 2 hours. 416 g (2.21 mol) of triisopropylborate was dropped into the reaction mixture at minus 60 degrees C. The reaction mixture was then stirred at room temperature for 17 hours. The reaction mixture was added with aqueous solution of hydrochloric acid and stirred at room temperature for 1 hour. After the reaction, the reaction mixture was added with toluene, and aqueous phase thereof was eliminated. Then, organic phase thereof was dried with magnesium sulfate, and the solvent was distilled away under reduced pressure. By recrystallizing the obtained solid by toluene, 126 g of 4-(1-naphthyl)phenylboronic acid was obtained at an yield of 67%.
References:
US2010/331585,2010,A1 Location in patent:Page/Page column 96-97

5419-55-6
387 suppliers
$12.00/5g

204530-94-9
199 suppliers
$6.00/250mg

870774-25-7
226 suppliers
$6.00/1g