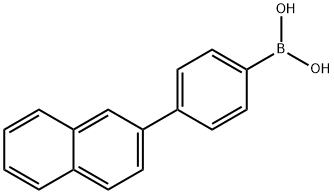
4-(NAPHTHALEN-2-YL)PHENYLBORONIC ACID synthesis
- Product Name:4-(NAPHTHALEN-2-YL)PHENYLBORONIC ACID
- CAS Number:918655-03-5
- Molecular formula:C16H13BO2
- Molecular Weight:248.08
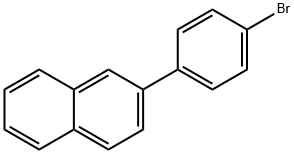
22082-99-1

918655-03-5
Under argon protection, 50.0 g (177 mmol) of 4-(2-naphthalene)bromobenzene was mixed with 500 mL of anhydrous tetrahydrofuran (THF) and cooled to -60 °C. Subsequently, 136 mL (212 mmol) of a hexane solution of 1.56 M n-butyllithium was pre-cooled to -60 °C and slowly added dropwise to the reaction mixture under stirring. After the dropwise addition was completed, the reaction mixture continued to be stirred at -60 °C for 1 hour. Next, 99.6 g (529 mmol) of triisopropyl borate was slowly added dropwise at the same temperature. After completion of the dropwise addition, the reaction mixture was gradually warmed to room temperature and stirred continuously for 18 hours. After completion of the reaction, aqueous hydrochloric acid was added to the mixture and stirred for 1 hour at room temperature. After that, toluene was added for extraction and the aqueous phase was separated and discarded. The organic phase was dried with magnesium sulfate and concentrated under reduced pressure to remove the solvent. Finally, the resulting solid was purified by recrystallization from toluene to give 33.6 g of (4-(naphthalen-2-yl)phenyl)boronic acid in 84% yield.
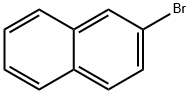
580-13-2
506 suppliers
$6.00/1g
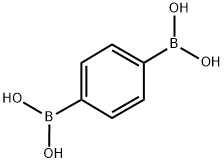
4612-26-4
305 suppliers
$6.00/1g

918655-03-5
220 suppliers
$22.00/250mg
Yield:918655-03-5 80%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in tetrahydrofuran;water at 80; for 2 h;
Steps:
2 synthesis of Intermediate B-1
2-bromo-naphthalene (2.07g, 10mmol), benzene-1,4-diBoronic acid(2.49g, 15mmol), Pd (PPh3) 4 (0.58 g, 0.5mmol), potassium carbonate (4.15g, 30mmol) and THF: H2O = 2:1 solution dissolved in 200ml 80 After 2 hours reflux agitation. Thereafter it was added and extracted three times with 40ml ethyl ether H2O40ml drying the obtained organic layer with magnesium sulfate, and separating the residue obtained by evaporating the solvent was subjected to silica gel column chromatography purification Intermediate B-1 (1.98g, 80% yield) It was obtained.
References:
Samsung Display Co., Ltd.;Kim, Su Youn;Kim, Hae Jin;Jon, Mi Uhn;Kim, Kwang Hyun;Kim, Young Guk;Hwang, Sok Hwan KR2016/30001, 2016, A Location in patent:Paragraph 0327-0330

22082-99-1
195 suppliers
$8.00/250mg

918655-03-5
220 suppliers
$22.00/250mg

121-43-7
381 suppliers
$13.00/25mL

22082-99-1
195 suppliers
$8.00/250mg

918655-03-5
220 suppliers
$22.00/250mg

5419-55-6
387 suppliers
$12.00/5g

22082-99-1
195 suppliers
$8.00/250mg

918655-03-5
220 suppliers
$22.00/250mg

5419-55-6
387 suppliers
$12.00/5g

22082-99-1
195 suppliers
$8.00/250mg

7732-18-5
507 suppliers
$12.69/100ml

918655-03-5
220 suppliers
$22.00/250mg