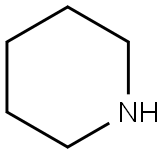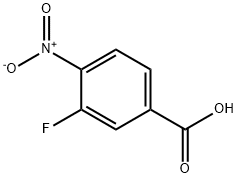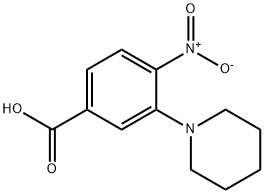
4-nitro-3-(piperidin-1-yl)benzoic acid synthesis
- Product Name:4-nitro-3-(piperidin-1-yl)benzoic acid
- CAS Number:1141473-72-4
- Molecular formula:C12H14N2O4
- Molecular Weight:250.25
Yield:1141473-72-4 91%
Reaction Conditions:
in ethanol at 20; for 24 h;
Steps:
18
3-Fluoro-4-nitrobenzoic acid (Combi-Blocks CA-4022, 5 g; 27.01 mmol) was dissolved in EtOH (30 mL) to which was added piperidine (8.02 mL; 81.03 mmol). The reaction mixture was stirred at RT for 24 hours, then concentrated and the residue was taken up in water (300 mL). The aqueous phase was washed with Et2O then the aqueous phase was acidified with acetic acid until pH 5. The aqueous phase was extracted with Et2O (900 mL). The combined organic phases were dried over MgSO4 and concentrated affording the title compound as an orange solid (6.18 g, 91 %). 1H NMR (DMSO-d6, 300 MHz) δ 13.55 (br s, 1 H), 7.89-7.86 (d, J = 8.42 Hz, 1 H), 7.77-7.76 (d, J = 1.40 Hz, 1 H), 7.60-7.57 (dd, J = 8.42 Hz, 1.50 Hz, 1 H), 3.23-2.98 (m, 2H), 3.21 -3.05 (m, 4H), 1.63-1 .58 (m, 6H). HPLC (Method A) Rt 3.88 min (Purity: 99.9%).
References:
WO2009/43889,2009,A2 Location in patent:Page/Page column 124