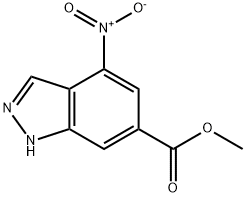
4-NITRO-6-INDAZOLECARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:4-NITRO-6-INDAZOLECARBOXYLIC ACID METHYL ESTER
- CAS Number:72922-61-3
- Molecular formula:C9H7N3O4
- Molecular Weight:221.17
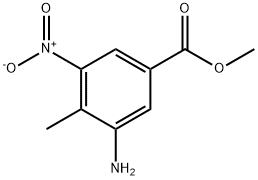
72922-60-2
29 suppliers
inquiry

72922-61-3
72 suppliers
$60.00/2mg
Yield:72922-61-3 22%
Reaction Conditions:
Stage #1: methyl 3-amino-4-methyl-5-nitrobenzoatewith hydrogenchloride;sodium nitrite in water at 0 - 20; for 1.5 h;
Stage #2: with sulfuric acid;urea in water at 50; for 0.25 h;
Steps:
197 Description 197; Methyl 4-NITRO-1H-INDAZOLE-6-CARBOXYLATE (D197)
To a suspension of 3-amino-4-methyl-5-nitrobenzoate (D196) (3.5 g, 16.7 mmol, 1 equiv) in H20 (100 ml) at 0°C was added 36% aqueous HCI solution (15 mi) and the resulting suspension was treated with NAN02 (1.35 g, 19.6 mmol, 1.2 equiv) then warmed to room temperature and stirred for 1.5 h. The insoluble material was removed by filtration and small amounts of urea were added to the mother liquors. The resulting solution was diluted with H20 (500 ml) and treated with H2SO4 (17.5 ML) then heated at 50°C for 15 min, cooled to room temperature and extracted with AcOEt. The organic phase was dried over MGS04 then concentrated in vacuo. The residue was triturated with MEOH to give methyl 4-nitro-1 H- indazole-6-carboxylate (D197) (0.8 g, 22%) as a cream solid which was used in the next step without further purification. [M+H] + = 222.1, RT = 2.84 min.
References:
WO2004/50619,2004,A1 Location in patent:Page 56
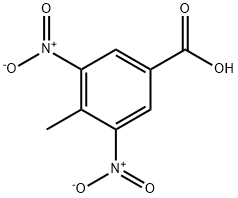
16533-71-4
220 suppliers
$9.00/10g

72922-61-3
72 suppliers
$60.00/2mg

54591-62-7
15 suppliers
inquiry

72922-61-3
72 suppliers
$60.00/2mg