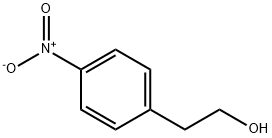
4-Nitrobenzeneethanol synthesis
- Product Name:4-Nitrobenzeneethanol
- CAS Number:100-27-6
- Molecular formula:C8H9NO3
- Molecular Weight:167.16
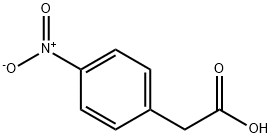
104-03-0

100-27-6
(1) Dissolve p-nitrophenylacetic acid in ether and heat under nitrogen protection to 50°C. Slowly add lithium aluminium hydride (mass ratio of lithium aluminium hydride to p-nitrophenylacetic acid 1:2) and make sure that the mixture is homogeneous. Subsequently, water was added dropwise (the mass ratio of the amount of water added dropwise to lithium aluminum hydride was 1:1) and the reaction continued to be stirred for 3 hours. Upon completion of the reaction, the reaction mixture was cooled to 0°C, and a 12% sodium hydroxide solution (the mass ratio of sodium hydroxide to lithium aluminum hydride was 3:2) and water (the mass ratio of water to lithium aluminum hydride for the second addition was 5:2) were slowly added. After standing for 20 minutes, it was gradually warmed to room temperature and dried by adding anhydrous MgSO4. After stirring for 30 minutes, filter and distill to remove the solvent in the filtrate to obtain p-nitrophenylethanol. (2) Dissolve p-nitrophenyl ethanol in ethanol and add catalyst (a mixture of CeO2, MnO and ZnO in the ratio of 1:2:5; the mass ratio of p-nitrophenyl ethanol to catalyst was 3:167). The reaction system was warmed up to 80°C under nitrogen atmosphere. hydrogen was introduced at atmospheric pressure and the reaction was carried out for 3 h. The reaction was carried out at atmospheric pressure and the reaction was carried out at atmospheric pressure and the reaction was carried out at atmospheric pressure. After completion of the reaction, it was cooled to room temperature and the catalyst was removed by filtration. Ethanol was removed by distillation and the resulting solid was purified by recrystallization to give p-aminophenethyl alcohol.

104-03-0
454 suppliers
$5.00/10g

100-27-6
259 suppliers
$6.00/5g
Yield:100-27-6 96.5%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether at 50; for 3 h;Inert atmosphere;Temperature;
Steps:
2 Example 2
(1) Dissolve p-nitrophenylacetic acid in ether.Heat to 50°C under nitrogen protectionAfter adding lithium aluminum hydride and mixing evenly,Add water, continue stirring for 3h,After the reaction is completed,Reduce the temperature to 0 °C,Add sodium hydroxide solution and water,The concentration of sodium hydroxide solution is 12%.After standing for 20 minutes, warm to room temperature and add anhydrous MgSO4.After stirring for 30min,The solvent in the filtrate is distilled off,Obtained p-nitrophenyl ethanol;(2) dissolving the obtained p-nitrophenyl ethanol in ethanol,The catalyst was added, nitrogen was introduced, and the temperature was raised to 80°C.Hydrogen gas is introduced under atmospheric pressureStir the reaction for 3h,Filter after cooling,Ethanol is distilled,After the resulting solid is recrystallized,Obtained p-aminophenyl ethanol;The catalyst is a mixture of CeO2, MnO, and ZnO.The mass ratio of lithium aluminum hydride used in step (1) to p-nitrophenylacetic acid is 1:2; the volume of water added dropwiseThe mass ratio to lithium aluminum hydride is 1:1; the mass ratio of sodium hydroxide to lithium aluminum hydride is 3:2 and the second additionThe mass ratio of water to lithium aluminum hydride is 5:2.The catalyst in step (2) is a mixture of CeO2, MnO and ZnO in a mass ratio of 1:2:5;The mass ratio of nitrobenzene ethanol is 3:167. The step (1)
References:
CN108033888,2018,A Location in patent:Paragraph 0019-0057
2945-08-6
67 suppliers
inquiry

100-27-6
259 suppliers
$6.00/5g
6388-74-5
36 suppliers
inquiry

100-27-6
259 suppliers
$6.00/5g
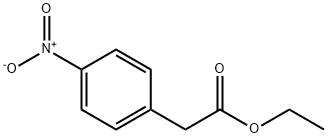
5445-26-1
175 suppliers
$10.00/1g

100-27-6
259 suppliers
$6.00/5g
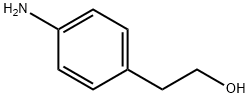
104-10-9
356 suppliers
$10.00/1g

100-27-6
259 suppliers
$6.00/5g