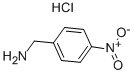
4-Nitrobenzylamine hydrochloride synthesis
- Product Name:4-Nitrobenzylamine hydrochloride
- CAS Number:18600-42-5
- Molecular formula:C7H9ClN2O2
- Molecular Weight:188.61
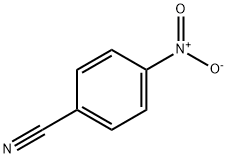
619-72-7

18600-42-5
General procedure for the synthesis of 4-nitrobenzylamine hydrochloride from p-nitrobenzonitrile: To a 6.0 mL solution of toluene containing p-nitrobenzonitrile (1.0 g, 5.1 mmol, 1.0 eq.) under nitrogen protection was added TMDS (900 μL, 5.1 mmol, 1.0 eq.) or PMHS (610 μL, 10.2 mmol, 2.0 eq.) and Ti(Oi-Pr)4 (1.5 mL, 5.1 mmol, 1.0 eq.). The reaction mixture was heated at 60 °C for 24 h, during which the colorless solution gradually changed to black, and the reaction progress could be monitored by TLC or 1H NMR. Upon completion of the reaction, the mixture was cooled to room temperature and acidified with 1 M aqueous HCl (7.7 mL, 1.5 eq.) to obtain a clarified solution. Subsequently, the crude product was concentrated under reduced pressure, and the resulting solid was filtered and washed with pentane (3 x 50 mL). The washed solid was dissolved in ethanol and finally the filtrate was concentrated under reduced pressure to give the target product 4-nitrobenzylamine hydrochloride.

619-72-7
381 suppliers
$11.00/1g

18600-42-5
149 suppliers
$9.00/250mg
Yield:18600-42-5 90%
Reaction Conditions:
Stage #1:4-nitrobenzonitrile with C46H178O41Si42;titanium(IV)isopropoxide in toluene at 60; for 9 h;Inert atmosphere;
Stage #2: with hydrogenchloride;water in toluene at 20;Inert atmosphere;chemoselective reaction;Reagent/catalyst;
Steps:
3 4.2. General procedure for the reduction of nitriles
General procedure: To a nitrogen purged screw-caped vial containing 1a (1.0 g, 5.1 mmol, 1.0 equiv) in 6.0 mL of toluene were added TMDS (900 μL, 5.1 mmol, 1.0 equiv) or PMHS (610 μL, 10.2 mmol, 2.0 equiv) and Ti(Oi-Pr)4 (1.5 mL, 5.1 mmol, 1.0 equiv) at rt. The mixture was then heated at 60 °C for 24 h (the colorless solution turned into black and the conversion of the substrate can be followed up by TLC and/or 1H NMR). After cooling to rt, the clear solution was acidified using aqueous 1 M HCl (7.7 mL, 1.5 equiv) and the crude mixture was concentrated under reduced pressure. The resulting solid was filtered, washed with pentane (3*50 mL), and dissolved in ethanol. The filtrate was finally concentrated under reduced pressure affording the amine 2a as a hydrochloride salt.
References:
Laval, Stéphane;Dayoub, Wissam;Pehlivan, Leyla;Métay, Estelle;Favre-Reguillon, Alain;Delbrayelle, Dominique;Mignani, Gérard;Lemaire, Marc [Tetrahedron,2014,vol. 70,# 4,p. 975 - 983] Location in patent:supporting information
619-80-7
22 suppliers
$13.00/5g

18600-42-5
149 suppliers
$9.00/250mg
17271-88-4
11 suppliers
inquiry

18600-42-5
149 suppliers
$9.00/250mg
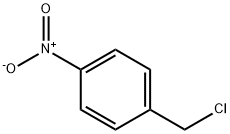
100-14-1
23 suppliers
$10.00/5g

18600-42-5
149 suppliers
$9.00/250mg
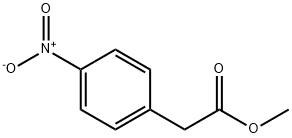
2945-08-6
66 suppliers
inquiry

18600-42-5
149 suppliers
$9.00/250mg