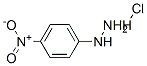
4-Nitrophenylhydrazine hydrochloride synthesis
- Product Name:4-Nitrophenylhydrazine hydrochloride
- CAS Number:636-99-7
- Molecular formula:C6H8ClN3O2
- Molecular Weight:189.6
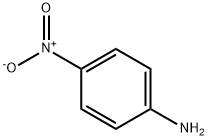
100-01-6
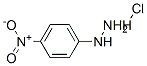
636-99-7
The general procedure for the synthesis of 4-nitrophenylhydrazine hydrochloride from 4-nitroaniline was as follows: 4-nitroaniline (1.47 g, 10.6 mmol) was dissolved in concentrated hydrochloric acid (2 mL) and the solution was cooled to 0°C. A solution of sodium nitrite (717 mg, 10.4 mmol) dissolved in water (4 mL) was added dropwise to the above reaction mixture under ice bath conditions with continuous stirring for 1 hour. Subsequently, a pre-cooled solution of concentrated hydrochloric acid (2 mL) of stannous chloride (4.7 g, 20.8 mmol) was slowly added to the diazo reaction mixture. The reaction mixture was continued to be stirred at 0°C for 2 hours. Upon completion of the reaction, the resulting yellow-orange precipitate was collected and washed thoroughly with ice-cold water until the filtrate was neutral (pH ≈ 7). Finally, the precipitate was dried under vacuum conditions overnight to afford the target product 4-nitrophenylhydrazine hydrochloride (640 mg, 4.18 mmol, 39% yield). The structure of the product was confirmed by 1H NMR (DMSO-d6): δ 4.49 (s, 2H), 6.78 (d, J=8.78 Hz, 2H), 7.98 (d, J=9.29 Hz, 2H), 8.41 (s, 1H).

100-01-6
72 suppliers
$11.19/25G
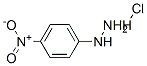
636-99-7
263 suppliers
$5.00/5g
Yield:636-99-7 39%
Reaction Conditions:
Stage #1:4-nitro-aniline with hydrogenchloride;sodium nitrite in water at 0; for 1 h;
Stage #2: with hydrogenchloride;tin(ll) chloride in water at 0; for 2 h;
Steps:
4.1.17 (4-Nitrophenyl)hydrazine, hydrochloride salt (26)
p-Nitroaniline (25) (1.47 g, 10.6 mmol) was dissolved in concd HCl (2 mL) and cooled to 0 °C. A solution of sodium nitrite (717 mg, 10.4 mmol) in ice-cooled water (4 mL) was added drop wise to above reaction mixture and stirred for 1 h in an ice bath. Ice-cooled solution of stannous chloride (4.7 g, 20.8 mmol) in concd HCl (2 mL) was added slowly to above diazonium reaction mixture. The reaction was stirred at 0 °C for 2 h. A yellow-orange precipitate was collected and washed thoroughly with ice-cold water until the filtrate pH was neutral. The precipitate was dried overnight under vacuum to give compound 26 (640 mg, 4.18 mmol, 39%); 1H NMR (DMSO-d6) δ 4.49 (s, 2H), 6.78 (d, J = 8.78 Hz, 2H), 7.98 (d, J = 9.29 Hz, 2H), 8.41 (s, 1H).
References:
Purohit, Meena K.;Chakka, Sai Kumar;Scovell, Iain;Neschadim, Anton;Bello, Angelica M.;Salum, Norue;Katsman, Yulia;Bareau, Madeleine C.;Branch, Donald R.;Kotra, Lakshmi P. [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 9,p. 2739 - 2752]
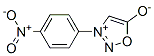
6299-51-0
0 suppliers
inquiry
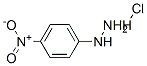
636-99-7
263 suppliers
$5.00/5g