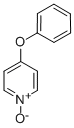
4-PHENOXYPYRIDINE-N-OXIDE synthesis
- Product Name:4-PHENOXYPYRIDINE-N-OXIDE
- CAS Number:33399-53-0
- Molecular formula:C11H9NO2
- Molecular Weight:187.19
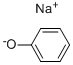
4783-86-2
102 suppliers
$8.00/1g

33399-53-0
3 suppliers
inquiry
Yield:33399-53-0 100%
Reaction Conditions:
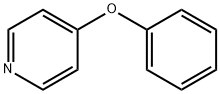
Stage #1: 4-phenoxypyridinewith 3-chloro-benzenecarboperoxoic acid in dichloromethane at 0; for 22 h;
Stage #2: with sodium hydrogencarbonate;sodium thiosulfate in dichloromethane;water at 20; for 0.166667 h;
Steps:
7
Preparation Example 7. C-(4-Phenoxy-pyridin-2-yl)-methylamine To a solution of 4-phenoxypyridine (3.0g, 17.5mmol) in dichloromethane (500mL) was added 3-chloro-perbenzoic acid (5.18g, 21.0mmol) on an ice bath, and the solution was stirred for 22 hours. An aqueous solution of saturated sodium thiosulfate and an aqueous solution of saturated sodium bicarbonate were added to the reaction solution, the solution was stirred at room temperature for 10 minutes, then, the organic layer was separated, washed with brine and dried over anhydrous magnesium sulfate. The solvent was evaporated, and 4-phenoxy-pyridine N-oxide (3.3g, quantitatively) was obtained as a pale yellow solid. The resulting solid (3.3g, 17.6mmol) was dissolved in acetonitrile (18mL), trimethylsilyl cyanide (6.6mL, 52.8mmol) and triethylamine (4.9mL, 35.2mmol) were added, and the solution was stirred for 5 hours under reflux. The solvent was evaporated, the residue was purified by silica gel column chromatography (hexane : ethyl acetate = 4 : 1), and 4-phenoxy-pyridine-2-carbonitrile (2.5g, 73%) was obtained as a pale yellow solid. Next, to a solution of lithium aluminum hydride (725mg,19.1mmol) in tetrahydrofuran (6.0mL) was portionwise added a solution of the resulting 4-phenoxy-pyridine-2-carbonitrile (1.5g, 7.65mmol) in tetrahydrofuran (3mL) on an ice bath, and the solution was stirred at room temperature for 15 hours. A mixture solvent of methanol and water (9:1) was added to the reaction solution, an aqueous solution of saturated ammonium chloride was further added, the solution was extracted with ethyl acetate, dried over anhydrous magnesium sulfate, then, the solvent was evaporated to obtain the title compound (730mg, 48%) as a pale brown oil. This was used in the next reaction without further purification.
References:
EP1782811,2007,A1 Location in patent:Page/Page column 68-69
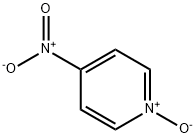
1124-33-0
336 suppliers
$10.00/1g

108-95-2
786 suppliers
$14.00/25g
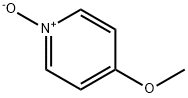
1122-96-9
180 suppliers
$22.00/1g

33399-53-0
3 suppliers
inquiry