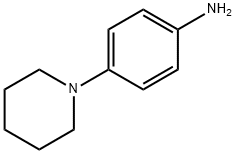
4-Piperidinoaniline synthesis
- Product Name:4-Piperidinoaniline
- CAS Number:2359-60-6
- Molecular formula:C11H16N2
- Molecular Weight:176.26
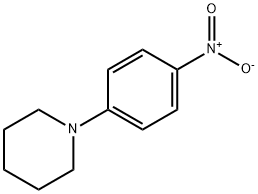
6574-15-8

2359-60-6
The general procedure for the synthesis of 1-(4-nitrophenyl)piperidine was as follows: first, 4-fluoronitrobenzene (323 mg, 2.3 mmol) was dissolved in DMSO (5 mL), followed by the addition of potassium carbonate (475 mg, 3.5 mmol) and piperidine (460 μL, 4.6 mmol). The reaction mixture was stirred at 90 °C for 9 hours. After completion of the reaction, water was added to the reaction solution and extracted twice with ethyl acetate. The organic layers were combined and washed twice with saturated aqueous NaCl followed by drying with anhydrous Na2CO3. The solvent was evaporated under reduced pressure to give 1-(4-nitrophenyl)piperidine (Y197, yield: 472 mg, 100%). Next, Y197 (472 mg) was dissolved in ethyl acetate (20 mL), Pd/C (186 mg) was added, and the reaction was stirred for 3 h at room temperature under hydrogen atmosphere. Upon completion of the reaction, the reaction mixture was filtered through diatomaceous earth and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: chloroform:methanol = 40:1) to afford 1-(4-aminophenyl)piperidine (Y222, yield: 394 mg, quantitative). Finally, Y491 (80 mg, 0.18 mmol) was dissolved in dichloromethane (2 mL), Y222 (100 mg, 0.58 mmol) was added and stirred at room temperature for 5 hours. After the reaction was completed, the solvent was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (eluent: chloroform:methanol = 35:1) to afford the target product 1-(p-aminophenyl)piperidine (yield: 68 mg, 64%). The structure of the product was confirmed by 1H NMR, 13C NMR and HRMS.1H NMR (500 MHz, CDCl3) δ 8.40 (s, 1H), 8.0 (d, 1H, J = 8.0 Hz), 7.70 (d, 1H, J = 8.5 Hz), 7.52 (dd, 1H, J = 8.0, 7.5 Hz), 6.92 (dd, 2H, J = 9.0, 3.5 Hz), 6.92 (dd, 2H, J = 9.0, 3.5 Hz). = 9.0, 3.5 Hz), 6.77 (dd, 2H, J = 9.0, 6.5 Hz), 5.33 (t, 1H, J = 6.0 Hz), 4.07 (bs, 2H), 3.11-3.09 (m, 4H), 2.79-2.64 (m, 4H), 1.69-1.53 (m, 10H), 1.43 (s, 9H), 1.09-1.01 (m, 9H) 1.09-1.01 (m, 2H).13C NMR (125 MHz, CDCl3) δ 154.9, 141.6, 140.8, 131.4, 130.9, 129.8, 125.8, 125.7, 79.7, 77.4, 48.7, 36.6, 29.6, 28.6, 25.7, 24.2.HRMS ( FAB-) m/z: [M-H]- calculated value C28H39N4O6S2, 591.2311; measured value, 591.2324.

6574-15-8
101 suppliers
$10.00/1g

2359-60-6
0 suppliers
$14.14/1gm:
Yield: 100%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in ethyl acetate at 20; for 3 h;
Steps:
2
4-Fluoronitrobenzene (323 mg, 2.3 mmol) was dissolved in DMSO (5 ml), potassium carbonate (475 mg, 3.5 mmol) and piperidine (460 μl, 4.6 mmol) were added, and the mixture was stirred at 90° C. for 9 hr.
Then, water was added to the reaction solution, and the mixture was extracted twice with ethyl acetate.
The organic layer was washed twice with saturated aqueous NaCl.
The organic layer was dried over Na2CO3, the solvent was evaporated to give compound Y197 (yield; 472 mg, 100%).
Compound Y197 was dissolved in ethyl acetate (20 ml), Pd/C (186 mg) was added, and the mixture was stirred under a hydrogen atmosphere at room temperature for 3 hr.
Then, the reaction solution was filtered through celite, the filtrate was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (eluent; chloroform:methanol (40:1)) to give compound Y222 (yield, quantitative, 394 mg).
Compound Y491 (mentioned later) (80 mg, 0.18 mmol) was dissolved in dichloromethane (2 ml), compound Y222 (100 mg, 0.58 mmol) was added, and the mixture was stirred at room temperature for 5 hr.
Then, the solvent was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (eluent; chloroform:methanol (35:1)) to give the title compound (yield; 68 mg, 64%).
1H NMR (500 MHz, CDCl3) δ8.40 (s, 1H), 8.0 (d, 1H, J=8.0 Hz), 7.70 (d, 1H, J=8.5 Hz), 7.52 (dd, 1H, J=8.0, 7.5 Hz), 6.92 (dd, 2H, J=9.0, 3.5 Hz), 6.77 (dd, 2H, J=9.0, 6.5 Hz), 5.33 (t, 1H, J=6.0 Hz), 4.07 (bs, 2H), 3.11-3.09 (m, 4H), 2.79-2.64 (m, 4H), 1.69-1.53 (m, 10H), 1.43 (s, 9H), 1.09-1.01 (m, 2H)
13C NMR (125 MHz, CDCl3) δ154.9, 141.6, 140.8, 131.4, 130.9, 129.8, 125.8, 125.7, 79.7, 77.4, 48.7, 36.6, 29.6, 28.6, 25.7, 24.2
HRMS (FAB-) m/z: [M-H]- calcd for C28H39N4O6S2, 591.2311. found, 591.2324
References:
KYOTO UNIVERSITY;Kakeya, Hideaki;Hattori, Akira;Takasu, Yasuaki;Fujii, Nobutaka;Oishi, Shinya;Kojima, Soichi;Hara, Mitsuko US2013/45977, 2013, A1 Location in patent:Paragraph 0211; 0212; 0213; 0214
110-89-4
0 suppliers
$15.96/100ML
106-47-8
481 suppliers
$5.00/5 g

2359-60-6
0 suppliers
$14.14/1gm:

110-89-4
0 suppliers
$15.96/100ML
106-40-1
483 suppliers
$12.00/5g

2359-60-6
0 suppliers
$14.14/1gm:
350-46-9
522 suppliers
$10.60/1gm:

2359-60-6
0 suppliers
$14.14/1gm:

110-89-4
0 suppliers
$15.96/100ML

2359-60-6
0 suppliers
$14.14/1gm: