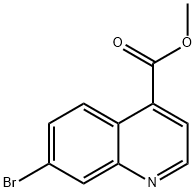
4-Quinolinecarboxylic acid, 7-broMo-, Methyl ester synthesis
- Product Name:4-Quinolinecarboxylic acid, 7-broMo-, Methyl ester
- CAS Number:220844-76-8
- Molecular formula:C11H8BrNO2
- Molecular Weight:266.09

67-56-1
790 suppliers
$7.29/5ml-f
31009-04-8
40 suppliers
$79.00/100mg

220844-76-8
33 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:methanol;7-bromo-4-quinoline-carboxylic acid with thionyl chloride for 2 h;Heating / reflux;
Stage #2: with water;sodium carbonate in methanol;ethyl acetate
Steps:
4.4a
4a) Methyl 7-bromo-4-quinolinecarboxylate To a solution of 750 mg (2.31 mmol) of dimethyl 7-bromo-2,4-quinolinedicarboxylate in a mixture of 20 mL of EtOH, 10 mL THF and 4 mL of H2O was added 925 mg (23.1 mmol) of NaOH. The mixture was stirred at 50° C. An additional 10 mL of THF and 15 mL of H2O were added after 5 min. After 30 min total at 50° C., the solution was concentrated to volume and the pH was adjusted to 4.0 with 1.0 N HCl (aq) followed by the addition of 100 mL of H2O. The resulting solids were collected by suction filtration, washed with H2O and dried. The solids were then added to 10 mL of diphenyl ether and the mixture stirred at 215° C. for 20 min. Upon cooling, 20 mL of hexanes was added and the resulting solids were collected by suction filtration, washed with hexanes and dried. The solids were then suspended in 20 mL of MeOH and 505 μL (6.90 mmol) of thionyl chloride was added. The mixture was refluxed for 1 hr, then an additional 505 μL (6.90 mmol) of thionyl chloride was added. After an additional 1 hr of refluxing, the solvent was evaporated. The residue was diluted with EtOAc then washed with saturated Na2CO3 (aq) and brine then dried over Na2SO4. Concentration of the solution yielded 320 mg (52%) of methyl 7-bromo-4-quinolinecarboxylate as a beige solid. 1H NMR (400 MHz, CDCl3): δ 9.01 (d, J=4 Hz, 1H), 8.70 (d, J=9 Hz, 1H), 8.35 (s, 1H), 7.92 (d, J=4 Hz, 1H), 7.75-7.72 (m, 1H), 4.03 (s, 3H). ESI-LCMS m/z 267 (M+H)+.
References:
SmithKline Beecham Corporation US2008/96921, 2008, A1 Location in patent:Page/Page column 37-38