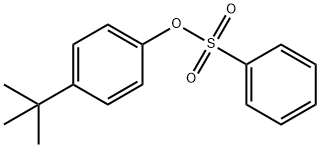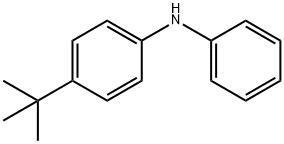
(4-TERT-BUTYL-PHENYL)-PHENYL-AMINE synthesis
- Product Name:(4-TERT-BUTYL-PHENYL)-PHENYL-AMINE
- CAS Number:4496-49-5
- Molecular formula:C16H19N
- Molecular Weight:225.33
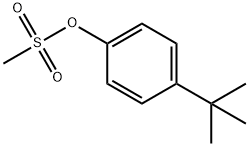
59970-38-6
5 suppliers
$57.00/50mg

62-53-3
689 suppliers
$10.00/1g

4496-49-5
77 suppliers
$46.00/250mg
Yield: 99%
Reaction Conditions:
with water;potassium carbonate;palladium diacetate;dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine in tert-butyl alcohol at 110; for 4 h;Product distribution / selectivity;Inert atmosphere;
Steps:
13
EXAMPLE THIRTEEN: Experimental Procedures for Reactions Described in Table 4, Figure 8; General Procedure Using the Precatalysts An oven-dried test tube, which was equipped with a magnetic stir bar and fitted with a teflon septum, was charged with the precatalyst (1 mol%), ligand (1 mol%), 4-t- butylphenyl methanesulfonate (0.5 mmol, 114 mg), and K2CO3 (97 mg, 0.7 mmol). The vessel was evacuated and backfilled with argon (this process was repeated a total of 3 times) and then the aniline (55 μL, 0.6 mmol) and tert-butanol (6 mL) were added via syringe. The solution was heated to 110 0C for 4 h, cooled to room temperature, diluted with Ethyl acetate, and washed with water. Dodecane was then added as an internal standard and the reaction was analyzed by GC.General Procedure for Water-Mediated Catalyst PreactivationAn oven-dried test tube, which was equipped with a magnetic stir bar and fitted with a teflon septum, was charged with Pd(OAc)2 (1 mol%) and ligand (3 mol%). The vessel was evacuated and backfilled with argon (this process was repeated a total of 3 times) and the tert-butanol (1 mL) and degassed H2O (8 mol%) were added via syringe. After addition of the water, the solution was heated to 110 0C for 1 min.A second oven-dried test tube, which was equipped with a magnetic stir bar and fitted with a Teflon septum, was charged with 4-t-butylphenyl methanesulfonate (0.5 mmol, 114 mg) and K2CO3 (97 mg, 0.7 mmol). The vessel was evacuated and backfilled with argon (this process was repeated a total of 3 times) and then the aniline (55 μL, 0.6 mmol) and tert-butanol (5 mL) were added via syringe and the activated catalyst solution was transferred from the first reaction vessel into the second via cannula. The solution was heated to 110 0C for 4 h, cooled to room temperature, diluted with Ethyl acetate, and
References:
MASSACHUSETTS INSTITUTE OF TECHNOLOGY WO2009/76622, 2009, A2 Location in patent:Page/Page column 123-124
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

62-53-3
689 suppliers
$10.00/1g

4496-49-5
77 suppliers
$46.00/250mg
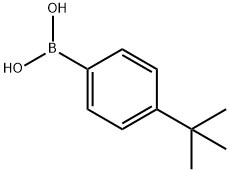
123324-71-0
410 suppliers
$5.00/1g

62-53-3
689 suppliers
$10.00/1g

4496-49-5
77 suppliers
$46.00/250mg