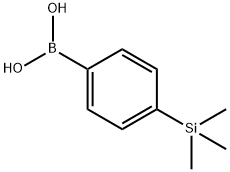
4-(TRIMETHYLSILYL)PHENYLBORONIC ACID synthesis
- Product Name:4-(TRIMETHYLSILYL)PHENYLBORONIC ACID
- CAS Number:17865-11-1
- Molecular formula:C9H15BO2Si
- Molecular Weight:194.11
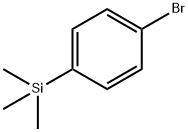
6999-03-7

17865-11-1
General procedure for the synthesis of 4-trimethylsilylphenylboronic acid from 1-bromo-4-trimethylsilylbenzene: 1-bromo-4-(trimethylsilyl)benzene (25 g, 0.10 mol) was placed in a 1-bulb flask and operated under argon protection. After addition of tetrahydrofuran (500 mL), the reaction mixture was cooled to -78 °C and stirred for 10 min. Slowly n-butyllithium (2.5 M hexane solution, 43.6 mL, 0.10 mol) was added dropwise and stirring was continued for 1 hour and 30 minutes at a maintained temperature of -78 °C. Subsequently, trimethyl borate (13.6 mL, 0.11 mol) was added at the same temperature. The reaction mixture was stirred at -78 °C for 30 minutes and then gradually warmed up to room temperature and continued stirring for 4 hours. After completion of the reaction, extraction was carried out with distilled water and ethyl acetate. The organic layer was dried with anhydrous magnesium sulfate and the solvent was subsequently removed by rotary evaporator. Finally, the target product 4-trimethylsilylphenylboronic acid (18 g, 85% yield) was purified by column chromatography using hexane and ethyl acetate as eluents.

6999-03-7
124 suppliers
$14.00/1g

17865-11-1
175 suppliers
$8.00/250mg
Yield:17865-11-1 85%
Reaction Conditions:
Stage #1:1-bromo-4-(trimethylsilyl)benzene with n-butyllithium in tetrahydrofuran;hexane at -78; for 1.5 h;Inert atmosphere;
Stage #2: with Trimethyl borate in tetrahydrofuran;hexane at -78 - 20;
Stage #3: with water in tetrahydrofuran;hexane;ethyl acetate
Steps:
1
l-Bromo-4-(trimethylsilyl)benzene (25 g, 0.10 mol) was added to a 1-bulb flask and an argon atmosphere was established. After adding tetrahydrofuran (500 mL), the mixture was stirred at -78 0C for 10 minutes. After adding n-BuLi (2.5 M in hexane, 43.6 mL, 0.10 mol) dropwise, the mixture was stirred for 1 hour and 30 minutes at -78 0C. Then, trimethyl borate (13.6 mL, 0.11 mol) was added at -78 0C. After stirring for 30 minutes at -78 0C, the mixture was stirred at room temperature for 4 hours. After the reaction was completed, extraction was performed using distilled water and EA. The organic layer was dried with MgSO4 and the solvent was removed using a rotary evaporator. Pure Compound A (18 g, 85%) was separated by column chromatography using hexane and EA as an eluent.
References:
DOW ADVANCED DISPLAY MATERIALS, LTD.;YOON, Seung Soo;KIM, Sung Min;KIM, Bong Ok;KWON, Hyuck Joo;EUM, Sung Jin;CHO, Young Jun;LEE, Hyo Jung WO2010/140801, 2010, A1 Location in patent:Page/Page column 18

5419-55-6
387 suppliers
$12.00/5g
106-37-6
479 suppliers
$9.00/5g

17865-11-1
175 suppliers
$8.00/250mg
75-77-4
592 suppliers
$5.04/10

5419-55-6
387 suppliers
$12.00/5g

106-37-6
479 suppliers
$9.00/5g

17865-11-1
175 suppliers
$8.00/250mg