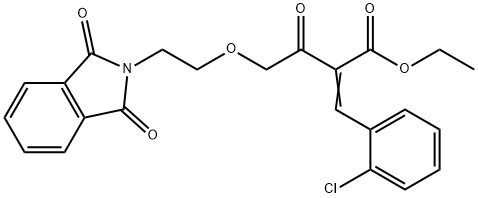
2-[(2-Chlorophenyl)Methylene]-4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxobutanoic Acid Ethyl Ester synthesis
- Product Name:2-[(2-Chlorophenyl)Methylene]-4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxobutanoic Acid Ethyl Ester
- CAS Number:400024-08-0
- Molecular formula:C23H20ClNO6
- Molecular Weight:441.86
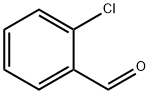
89-98-5
669 suppliers
$15.00/25g
![ETHYL4-[2-(1,3-DIOXO-1,3-DIHYDRO-2H-ISOINDOL-2-YL)ETHOXYL]-3-OXOBUTANOATE](/CAS/GIF/88150-75-8.gif)
88150-75-8
149 suppliers
$85.00/100mg
![2-[(2-Chlorophenyl)Methylene]-4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxobutanoic Acid Ethyl Ester](/CAS/20150408/GIF/400024-08-0.gif)
400024-08-0
27 suppliers
inquiry
Yield:400024-08-0 56%
Reaction Conditions:
with pyrrolidine;acetic acid in isopropyl alcohol at 37; for 5 h;
Steps:
11 EXAMPLE 11; Preparation of ethyl-2-(2-chlorobenzylidine)-4-[2-(phthalimido)ethoxy]acetoacetate (XVIII)
Preparation of ETHYL 2-(2-CHLOROBENZYLIDINE)-4-[2-(PHTHALIMIDE) ETHOXY] ACETOACETATE (XV : A 250 mL two-necked flask, equipped with a magnetic stirring bar and thermometer was charged with 10 g of ethyl 4- [2- (phthalimido) ethoxy] acetoacetate and 3.96 g 2- chlorobenzaldehyde and 100 mL isopropanol under nitrogen atmosphere. To the resulting mixture was added 0.188 ML of acetic acid and 0.223 ML pyrolidine. The reaction mixture was heated to 37 °C and stirred at this temperature for 5 hours. The solvent was removed in vacuo and the residue was dissolved in 100 ML of methylene chloride. The resulting solution was washed with 100 mL of sat. NAHCO3 and 100 mL of water, dried over 5 G OF MGS04. MGS04 was filtered off, the filtrate was concentrated in vacuo. The residue was purified by flash chromatography using ethyl acetate/hexane (20/80) as an eluent to give 7.72 g of ethyl 2- (2-CHLOROBENZYLIDINE)-4- [2- (PHTHALIMIDE) ethoxy] acetoacetate as an light brown oil (yield 56%, rel. compound purity > 98%). 1H-NMR (CDCL3) No. 7.89 (s, 1H), 7.78 (dd, 2H), 7.69 (dd, 2H), 7.35 (M, 1H), 7.28 (M, 2H), 7.17 (M, 1H), 4.44 (s, 2H), 4.05 (Q, 2H), 3.76 (T, 2H), 3.64 (t, 2H), 1.20 (t, 3H). 13C-NMR (CDCI3) 6 203. 2,168. 4,164. 0,140. 0,134. 0,133. 9,132. 3,131. 5, 130.4, 129.8, 127.3, 126.8, 123.4, 77.7, 68.5, 62.0, 37.4, 14.3.
References:
WO2004/58711,2004,A1 Location in patent:Page 20
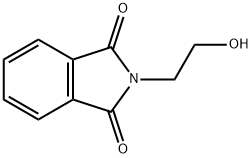
3891-07-4
334 suppliers
$6.00/10g
![2-[(2-Chlorophenyl)Methylene]-4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxobutanoic Acid Ethyl Ester](/CAS/20150408/GIF/400024-08-0.gif)
400024-08-0
27 suppliers
inquiry
85-44-9
780 suppliers
$9.00/5g
![2-[(2-Chlorophenyl)Methylene]-4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxobutanoic Acid Ethyl Ester](/CAS/20150408/GIF/400024-08-0.gif)
400024-08-0
27 suppliers
inquiry
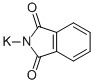
1074-82-4
502 suppliers
$5.00/25g
![2-[(2-Chlorophenyl)Methylene]-4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxobutanoic Acid Ethyl Ester](/CAS/20150408/GIF/400024-08-0.gif)
400024-08-0
27 suppliers
inquiry
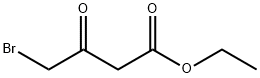
13176-46-0
168 suppliers
$8.00/250mg
![2-[(2-Chlorophenyl)Methylene]-4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxobutanoic Acid Ethyl Ester](/CAS/20150408/GIF/400024-08-0.gif)
400024-08-0
27 suppliers
inquiry