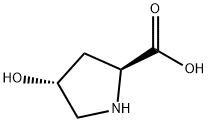
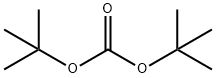
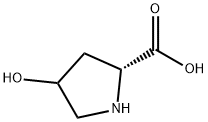
1-(tert-butoxycarbonyl)-4-hydroxypyrrolidine-2-carboxylic acid synthesis
- Product Name:1-(tert-butoxycarbonyl)-4-hydroxypyrrolidine-2-carboxylic acid
- CAS Number:40350-82-1
- Molecular formula:C10H17NO5
- Molecular Weight:231.25
Yield:40350-82-1 100%
Reaction Conditions:
in sodium hydroxide;water;tert-butyl alcohol;
Steps:
1.A A.
A. N-t-Butoxycarbonyl-trans-4-hydroxy-L-proline 4-Hydroxyproline (31.2 g. 0.238 mole) was dissolved in sodium hydroxide (9.5 g) in water (75 ml) and t-butanol (50 ml) and to that was added with stirring and ice-cooling, a solution of di-tert-butyl-dicarbonate (57.1 g, 0.262 mole) in t-butanol (50 ml) and the stirring was continued overnight at ambient temperature. Water (125 ml) was added to the reaction mixture and was extracted with hexanes (2*100 ml). The aqueous layer was acidified with KHSO4 (~35 g in 150 ml H2 O) in an ice bath to a pH~2 or 3. It was extracted with ethylacetate (4*100 ml) and the combined organic phase was washed with brine and dried over anhydrous sodium sulfate. Removal of the solvent on a rotavap gave a glassy solid in 100% yield, m.p. 124° C. This material was used for the next step without any further purification. Anal. Calc'd for C10 H17 O5 N: C, 51.95; H, 7.36; N, 6.06. Found: C, 51.67; H, 7.37; N, 5.92.
References:
US4501901,1985,A