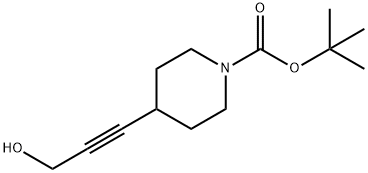
4-(3-Hydroxy-prop-1-ynyl)-piperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:4-(3-Hydroxy-prop-1-ynyl)-piperidine-1-carboxylic acid tert-butyl ester
- CAS Number:403802-41-5
- Molecular formula:C13H21NO3
- Molecular Weight:239.31
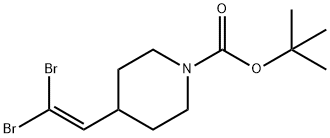
203664-61-3

403802-41-5
General procedure for the synthesis of tert-butyl 4-(3-hydroxyprop-1-yn-1-yl)piperidine-1-carboxylate from tert-butyl 4-(2,2-dibromoethenyl)piperidine-1-carboxylate: 6.0 g (16.3 mmol) of tert-butyl 4-(2,2-dibromoethenyl)piperidine-1-carboxylate was dissolved in 150 ml of THF at -78 °C and 21.4 ml ( 34.2 mmol) n-butyllithium (~1.6 M hexane solution) was added slowly. After keeping the reaction at this temperature for 2 hours, 4.9 g (16.3 mmol) of paraformaldehyde was added. The reaction mixture was then slowly warmed to room temperature and the reaction continued for 3 hours. Upon completion of the reaction, the mixture was partitioned (3 times) between water and ether. The organic phase was washed with 10% NaCl aqueous solution, dried over anhydrous Na2SO4 and then concentrated. Purification by rapid chromatography on silica gel (hexane/EtOAc 4:1) afforded 3.34 g (86% yield) of the target product, tert-butyl 4-(3-hydroxyprop-1-yn-1-yl)piperidine-1-carboxylate, with mass spectrometry (MS) revealing the molecular ion peak at 239 (M).

50-00-0
896 suppliers
$10.00/25g

203664-61-3
32 suppliers
$90.00/250mg

403802-41-5
24 suppliers
$645.00/1g
Yield:403802-41-5 74%
Reaction Conditions:
Stage #1: tert-butyl 4-(2,2-dibromovinyl)piperidine-1-carboxylatewith n-butyllithium in tetrahydrofuran;hexane at -45; for 0.75 h;
Stage #2: formaldehyd in tetrahydrofuran;hexane at -45 - 20;
Steps:
[00231] Step 2: tert-butyl 4-(3-hydroxyprop-1-yn-1-yl)piperidine-1-carboxylate
[00232] To a solution of tert-butyl 4-(2,2-dibromovinyl)piperidine-1-carboxylate (7.84 g, 21.2 mmol) in THF (100 mL) cooled at -45oC was added n-butyl lithium (17.4 m of a 2.5M soln in Hexanes, 43.5 mmol) slowly over 10 mins. After complete addition mixture stirred at -45oC for 45 minutes then paraformaldehyde (1.91 g, 63.6 mmol) added and mixture allowed to warm slowly to warm to room temperature and stirred overnight. Mixture quenched by the addition of sat.NH4Cl (200 mL) and extracted with EtOAc (300 mL); organic layer washed with water (200 mL), sat. NaCl (100 mL), dried over MgSO4, filtered and evaporated. The residue was purified by silica gel column chromatography (Teledyne Isco: SNAP 80g GOLD) eluent: gradient 0-100% EtOAc in Heptane (7cv) to give tert-butyl 4-(3-hydroxyprop-1-yn-1-yl)piperidine-1-carboxylate (3.77 g, 74%) as a light yellow oil..1H NMR (500 MHz, Chloroform-d) δ 4.27 (dd, J = 6.0, 2.0 Hz, 2H), 3.75 - 3.66 (m, 2H), 3.14 (ddd, J = 13.5, 8.8, 3.4 Hz, 2H), 2.60 (ttq, J = 8.2, 4.0, 2.0 Hz, 1H), 1.77 (ddt, J = 13.7, 6.3, 3.5 Hz, 2H), 1.56 (dtt, J = 12.7, 8.6, 3.7 Hz, 2H), 1.45 (s, 9H).
References:
WO2021/71837,2021,A1 Location in patent:Paragraph 00231-00232

203664-61-3
32 suppliers
$90.00/250mg

403802-41-5
24 suppliers
$645.00/1g