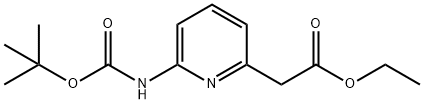
(6-TERT-BUTOXYCARBONYLAMINO-PYRIDIN-2-YL)-ACETIC ACID ETHYL ESTER synthesis
- Product Name:(6-TERT-BUTOXYCARBONYLAMINO-PYRIDIN-2-YL)-ACETIC ACID ETHYL ESTER
- CAS Number:408365-87-7
- Molecular formula:C14H20N2O4
- Molecular Weight:280.32
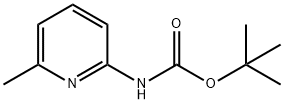
90101-22-7
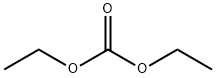
105-58-8

408365-87-7
The general procedure for the synthesis of ethyl [6-[(tert-butoxycarbonyl)amino]-2-pyridinyl]acetate from tert-butyl (6-methylpyridin-2-yl)carbamate and diethyl carbonate was as follows: to an anhydrous THF solution of tert-butyl (6-methylpyridin-2-yl)carbamate (0.97 g, 4.66 mmol) was slowly added, at -78 °C, LDA (1.8 M heptane solution, 4 equiv, 18.6 mmol) and the reaction mixture was stirred for 30 min. Subsequently, diethyl carbonate (2 eq., 9.32 mmol) was added and stirring was continued at -78 °C for 10 min. The reaction mixture was slowly warmed to 0 °C and stirring was continued at this temperature for 2 hours. Upon completion of the reaction, the reaction was quenched with saturated NH4Cl solution followed by extraction of the reaction mixture with ethyl acetate. The organic phases were combined, dried with anhydrous MgSO4 and concentrated under reduced pressure to remove the solvent. The crude product was purified by column chromatography with heptane/ethyl acetate as eluent (gradient from 98:2 to 95:5) to afford the target product [6-[(tert-butoxycarbonyl)amino]-2-pyridinyl]ethyl acetate (1.36 g in >100% yield, probably due to incomplete removal of trace solvent).

90101-22-7
76 suppliers
$42.60/1g

105-58-8
479 suppliers
$17.00/25g

408365-87-7
48 suppliers
$40.00/100mg
Yield:408365-87-7 100%
Reaction Conditions:
Stage #1: 2-(tert-butoxycarbonylamino)-6-picolinewith lithium diisopropyl amide in tetrahydrofuran;n-heptane at -78; for 0.5 h;
Stage #2: Diethyl carbonate in tetrahydrofuran;n-heptane at -78 - 0; for 2.16667 h;
Steps:
2.B; 4.H
2B. (β-tei't-Butoxycarbonylamino-pyridin-l-yl) acetic acid ethyl ester; To a solution of title compound 2A, (6-methyl-pyridin-2-yl) carbamic acid t?T-butyl ester, (0.97 g, 4.66 mmol) in anhydrous THF at -780C is added LDA (1.8 M solution in heptanes; 4 eq, 18.6 mmol) and the mixture is stirred for 30 min. Diethyl carbonate (2 eq, 9.32 mmol) is added and the mixture is stirred at -780C for a further 10 min. The mixture is allowed to warm to O0C and stirring continued for 2 h. The reaction is quenched with saturated NH4Cl solution. The solution is extracted with ethyl acetate. The combined organic phase is dried (MgSO4) and concentrated in vacuo. The crude product is purified by column chromatography with heptane/ ethyl acetate (gradient 98:2 - 95:5) to give the title compound (1.36 g, >100% repeated drying can not remove all traces of solvent)
References:
WO2006/50908,2006,A1 Location in patent:Page/Page column 32; 39-40
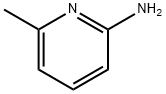
1824-81-3
486 suppliers
$8.00/25g

408365-87-7
48 suppliers
$40.00/100mg

24424-99-5
863 suppliers
$13.50/25G

408365-87-7
48 suppliers
$40.00/100mg