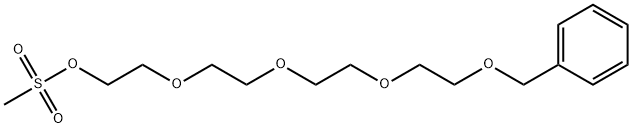
1-Phenyl-2,5,8,11-tetraoxatridecan-13-yl methanesulfonate synthesis
- Product Name:1-Phenyl-2,5,8,11-tetraoxatridecan-13-yl methanesulfonate
- CAS Number:477781-69-4
- Molecular formula:C16H26O7S
- Molecular Weight:362.44
![2-[2-[2-[2-(BENZYLOXY)ETHOXY]ETHOXY]ETHOXY]ETHANOL](/CAS/GIF/86259-87-2.gif)
86259-87-2
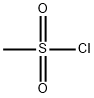
124-63-0

477781-69-4
1. To a stirred mixture of 1-phenyl-2,5,8,11-tetraoxatridecan-13-ol (45.1 g, 159 mmol) and triethylamine (62.2 g, 636 mmol) in dichloromethane (800 mL) at 0 °C was slowly added methanesulfonyl chloride (36.4 g, 318 mmol). 2. After addition, the reaction mixture was stirred at room temperature overnight. 3. Upon completion of the reaction, the reaction was quenched with 1N HCl (300 mL) and then extracted with ethyl acetate. 4. The organic phases were combined, dried over magnesium sulfate, concentrated to dryness, and purified by fast chromatography to afford 1-benzyloxytrioxaundecanemethanesulfonate (56.2 g, 98%) as a clear oil. 1H NMR (400MHz, CDCl3) δ 7.25-7.32 (m, 5H), 4.54 (s, 2H), 4.32-4.34 (m, 2H), 3.71-3.73 (m, 2H), 3.59-3.66 (m, 12H), 3.03 (s, 3H).
![2-[2-[2-[2-(BENZYLOXY)ETHOXY]ETHOXY]ETHOXY]ETHANOL](/CAS/GIF/86259-87-2.gif)
86259-87-2
143 suppliers
$24.00/1g

124-63-0
0 suppliers
$10.00/5g

477781-69-4
34 suppliers
$72.00/100mg
Yield:477781-69-4 100%
Reaction Conditions:
with triethylamine in dichloromethane at -5 - 20; for 18 h;Inert atmosphere;
Steps:
7.1 Step (1)
2-[2-[2-(2-benzyloxyethoxy)ethoxy]ethoxy]ethanol (50 g, 176 mmol) and triethylamine (35.6 g, 352 mmol) in dry dichloromethane (500 mL) under nitrogen were cooled to -5°C. Methanesulfonyl chloride (30.2 g, 264 mmol) in dry DCM (20 mL) was added dropwise to this solution at 0°C. The mixture was allowed to warm to room temperature and stirred at room temperature for 18 h. Tri-ethylamine hydrochloride was fdtered off, and the DCM solution was washed with 0.1 N HC1 and dried over sodium sulfate. Removing the solvent afforded 2-[2- [2-(2-benzyloxyethoxy)ethoxy] ethoxy] ethyl methanesulfonate (69.1 g, 175 mmol, quant.) as light yellow oil which was used without further purification. NMR (400 MHz, CDCh) d 7.37 - 7.27 (m, 5H), 4.56 (s, 2H), 4.39 - 4.33 (m, 2H), 3.78 - 3.73 (m, 2H), 3.69 - 3.60 (m, 12H), 3.06 (s, 3H).
References:
WO2022/13443,2022,A1 Location in patent:Page/Page column 89-91
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
371 suppliers
$7.00/25g

124-63-0
0 suppliers
$10.00/5g

477781-69-4
34 suppliers
$72.00/100mg
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
371 suppliers
$7.00/25g

477781-69-4
34 suppliers
$72.00/100mg
112-76-5
253 suppliers
$40.32/25G

477775-73-8
68 suppliers
inquiry

477781-69-4
34 suppliers
$72.00/100mg
100-39-0
429 suppliers
$10.00/10g

477781-69-4
34 suppliers
$72.00/100mg