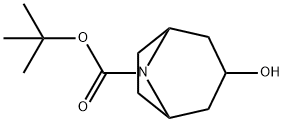
tert-Butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate synthesis
- Product Name:tert-Butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate
- CAS Number:478837-18-2
- Molecular formula:C12H21NO3
- Molecular Weight:227.3
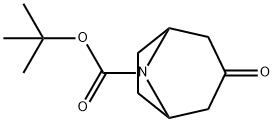
185099-67-6
![tert-Butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/20180629/GIF/478837-18-2.gif)
478837-18-2
The general procedure for the synthesis of tert-butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate using 3-oxo-8-azabicyclo[3.2.1]octane-8-carboxylic acid as a starting material was as follows: sodium borohydride (178 mg, 4.7 mmol) was slowly added to a solution of tert-butyl 3-oxo-8-azabicyclo[3.2.1]octane-8-carboxylate (0.50 g, 2.2 mmol) at room temperature. butyl ester (0.50 g, 2.2 mmol) in a solution of ethanol (10 mL). The reaction mixture was stirred continuously for 1 hour at room temperature. Upon completion of the reaction, the reaction was quenched with saturated ammonium chloride solution (30 mL) followed by extraction with ethyl acetate (3 x 20 mL). The organic phases were combined, washed sequentially with water and brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford the title compound tert-butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate (463 mg, 92% yield) as a mixture of endo and exo stereoisomers. The product was analyzed by GC-MS (EI) and the molecular ion peak (M+) of molecular formula C12H21NO3 was 227.

185099-67-6
246 suppliers
$5.00/250mg
![tert-Butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/20180629/GIF/478837-18-2.gif)
478837-18-2
42 suppliers
$21.00/250mg
Yield: 92%
Reaction Conditions:
with sodium tetrahydroborate;ethanol at 20; for 1 h;
Steps:
14.1
Sodium borohydride (178 mg, 4.7 mmol) was added to a solution of tert-butyl 3-oxo-8-azabicyclo[3.2.1]octane-8-carboxylate (0.50 g, 2.2 mmol) in ethanol (10 mL), and the resulting mixture was stirred at room temperature for one hour. The mixture was quenched with saturated ammonium chloride solution (30 mL), and extracted with ethyl acetate (3x 20 mL). The combined extract was washed with water then brine, dried over sodium sulfate, filtered and concentrated to give tert-butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate (463 mg, 92% yield) as a mixture of endo and exo stereoisomers. GC-MS (EI) for C12H21NO3: 227 (M+).
References:
EXELIXIS, INC.;AAY, Naing;BLAZEY, Charles, M.;BOWLES, Owen, Joseph;BUSSENIUS, Joerg;CURTIS, Jeffry, Kimo;DEFINA, Steven, Charles;DUBENKO, Larisa;HARRIS, Jason, R.;JACKSON-UGUETO, Eileen, E.;KIM, Angie, Inyoung;MANALO, Jean-claire, Limun;PACK, Michael;PETO, Csaba, J.;RICE, Kenneth, D.;TSANG, Tsze, H.;WANG, Longcheng WO2010/138490, 2010, A1 Location in patent:Page/Page column 33
![8-Azabicyclo[3.2.1]octan-3-ol](/CAS/20180601/GIF/7432-11-3.gif)
7432-11-3
8 suppliers
inquiry

24424-99-5
869 suppliers
$13.50/25G
![tert-Butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/20180629/GIF/478837-18-2.gif)
478837-18-2
42 suppliers
$21.00/250mg

24424-99-5
869 suppliers
$13.50/25G
![tert-Butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/20180629/GIF/478837-18-2.gif)
478837-18-2
42 suppliers
$21.00/250mg
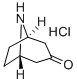
25602-68-0
209 suppliers
$11.00/5g
![tert-Butyl 3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate](/CAS/20180629/GIF/478837-18-2.gif)
478837-18-2
42 suppliers
$21.00/250mg