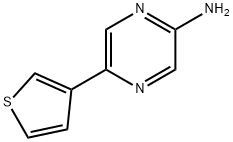
5-(3-THIENYL)-2-PYRAZINAMINE synthesis
- Product Name:5-(3-THIENYL)-2-PYRAZINAMINE
- CAS Number:710323-21-0
- Molecular formula:C8H7N3S
- Molecular Weight:177.23
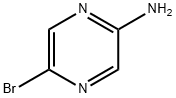
59489-71-3
398 suppliers
$5.00/1g
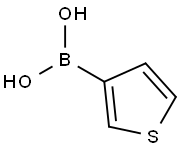
6165-69-1
342 suppliers
$12.72/1gm:

710323-21-0
12 suppliers
inquiry
Yield:710323-21-0 71%
Reaction Conditions:
with potassium carbonate;tetrakis-(triphenylphosphine)-palladium in DMF (N,N-dimethyl-formamide) at 100; for 16 h;
Steps:
5-(thior)hen-3-yl)pvrazin-2-amine (N); In a dry 100 mL round bottom flask, a mixture of M (2.30 g, 13 mmol), thiopherie-3-boronic acid (2.54 g, 20.0 mmol), Pd(PPh3)4 (2.29 g, 2.0 mmol) and K2C03 (5.47 g, 40.0 mmol) in DMF (40 mL) was bubbled with N2 for 10 min, then the mixture was stirred at 100 °C for 16 hours. The resulting mixture was evaporated to dryness. The residue was purified using column chromatography (silica gel, 2:1:7 hexane/CH2CI2/EtOAc). The fractions containing product were evaporated to dryness under vacuum to yield compound N as a light brown solid (1.63 g, 9.22 mmol, 71%). 1H NMR (DMSO-d6, 300 MHz) No. 6.46 (bs, 2H), 7.55-7.65 (m, 2H), 7.85 (bs, 1 H), 7.90 (d, 1 H, J, 1.4), 8.42 (d, 1 H, J, 1.4).
References:
WO2005/113548,2005,A1 Location in patent:Page/Page column 48