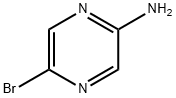
2-Amino-5-bromopyrazine synthesis
- Product Name:2-Amino-5-bromopyrazine
- CAS Number:59489-71-3
- Molecular formula:C4H4BrN3
- Molecular Weight:174
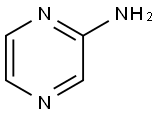
5049-61-6

59489-71-3
Example 1A Synthesis of 5-bromo-2-pyrazinamine: 2-Aminopyrazine (15.0 g, 157 mmol) was dissolved in dichloromethane (900 mL), N-bromosuccinimide (28.2 g, 159 mmol) was added, and the reaction was stirred for 3.5 hours at room temperature. After completion of the reaction, the reaction mixture was filtered through diatomaceous earth (Celite?). The filtrate was treated with silica gel (300 g) and subsequently concentrated under reduced pressure. The resulting crude product was purified by fast column chromatography with 30% ethyl acetate/hexane as eluent to give 22.09 g (81.5% yield) of the target compound 5-bromo-2-pyrazinamine. Mass spectrum (APCI(+)) m/z: 174 ([M + H]+); 1H NMR (300 MHz, CDCl3) δ: 8.09 (d, J = 1.4 Hz, 1H), 7.77 (d, J = 1.7 Hz, 1H), 4.30-4.78 (br s, 2H).

5049-61-6
444 suppliers
$8.00/10g

59489-71-3
396 suppliers
$5.00/1g
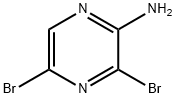
24241-18-7
439 suppliers
$6.00/5g
Yield:59489-71-3 88% ,24241-18-7 6%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 100; for 0.0833333 h;Microwave irradiation;Solvent;Temperature;
Steps:
Reaction under Microwave Irradiation; General Procedure
General procedure: To a solution of the pyrazine-2-amine (1.0 mmol) in acetonitrile (5 mL) in a glass tube (10 mL) sealed with a silicon septum was added N-iodosuccinimide (NIS) (procedure A) (1.1–3.0 mmol)or I2 (1.1 mmol) or N-chlorosuccinimide (NCS) (procedure B) orN-bromosuccinimide (NBS) (procedure C) (1.1–2.2 mmol) containing magnetic stirring. The tube was introduced to the microwave oven, heated to 100 °C, and subjected to a variable MW power until 300 W (an IR sensor measured the temperature of the glass tube surface) until no starting material couldbe detected by thin-layer chromatography. Then, the solvent was removed under reduced pressure to give the crude product,which was purified by silica gel column chromatography. Alternatively, the crude reaction mixture can be extracted with diethyl ether/water (3×15 mL), dried with sodium sulfate, filtered,and the solvent can be removed to dryness. Representative product:
References:
Grima, Josep;Lizano, Enric;Pujol, M. Dolors [Synlett,2019,vol. 30,# 17,p. 2000 - 2003]

5049-61-6
444 suppliers
$8.00/10g

59489-71-3
396 suppliers
$5.00/1g

5049-61-6
444 suppliers
$8.00/10g

59489-71-3
396 suppliers
$5.00/1g
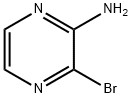
21943-12-4
136 suppliers
$60.00/250 mg

24241-18-7
439 suppliers
$6.00/5g

5049-61-6
444 suppliers
$8.00/10g

59489-71-3
396 suppliers
$5.00/1g

24241-18-7
439 suppliers
$6.00/5g

59489-71-3
396 suppliers
$5.00/1g