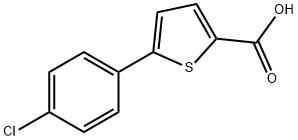
5-(4-Chlorophenyl)thiophene-2-carboxylic acid synthesis
- Product Name:5-(4-Chlorophenyl)thiophene-2-carboxylic acid
- CAS Number:40133-14-0
- Molecular formula:C11H7ClO2S
- Molecular Weight:238.69
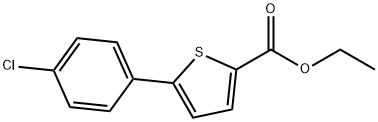
19282-40-7
7 suppliers
inquiry

40133-14-0
32 suppliers
$42.40/1mg:
Yield:40133-14-0 96%
Reaction Conditions:
Stage #1: ethyl 5-(4-chlorophenyl) thiophene-2-carboxylatewith sodium hydroxide;water in methanol; for 6 h;Heating / reflux;
Stage #2: with hydrogenchloride in methanol;water at 20; pH=3;
Steps:
9.B
A mixture of ethyl 5-bromothiophene-2-carboxylate (1.0 g, 4.25 mmol), 4-chlorophenylboronic acid (0.998 g, 6.38 mmol), 2.0 M aq NaHCO3 (6.38 ml, 12.76 mmol) and tetrakis(triphenylphosphine)palladium(0) (0.492 g, 0.425 mmol) in DMF (40 ml) under nitrogen was stirred at 100° C. under nitrogen in a sealed tube for 18 hours as described in WO 2007/011284. The reaction was then cooled to RT, diluted with saturated aq NaHCO3 (40 ml) and extracted with EtOAc (40 ml). The EtOAc extracts were dried over Na2SO4 and concentrated. The crude product was purified by ISCO chromatography on a silica gel column (120 g) employing a 10 min gradient ranging from hexane to 30% EtOAc to elute ethyl 5-(4-chlorophenyl)thiophene-2-carboxylate (1.05 g, 3.94 mmol, 93% yield) as a white solid. LC MS at t=2.70 min. (m+H=267) Phenomenex S5 C18 4.6×30 mm column/water-MeOH-TFA 90:10:0.1 to 10:90:0.1 gradient over 2 min at 5 mL/min with 1 min hold at the end of the gradient. 1H NMR (400 MHz, CD3OD) δ ppm 1.39 (t, J=7.18 Hz, 3H), 4.37 (q, J=7.22 Hz, 2H), 7.43-7.51 (m, 3H), 7.71 (d, J=8.56 Hz, 2H), 7.78 (d, J=4.03 Hz, 1H).A mixture of ethyl 5-(4-chlorophenyl)thiophene-2-carboxylate (1.05 g, 3.94 mmol), methanol (40 ml) and 1.0 M aq NaOH (15.75 ml, 15.75 mmol) was stirred at reflux for 6 hours. Upon cooling to RT, the reaction was acidified to pH 3 with conc. HCl and extracted with EtOAc (60 ml). The EtOAc layer was dried over Na2SO4 and concentrated to yield 5-(4-chlorophenyl)thiophene-2-carboxylic acid (902 mg, 3.78 mmol, 96% yield) as an off-white solid. LC MS at t=2.44 min. (m+H=240) Phenomenex S5 C18 4.6×30 mm column/water-MeOH-TFA 90:10:0.1 to 10:90:0.1 gradient over 2 min at 5 mL/min with 1 min hold at the end of the gradient. 1H NMR (400 MHz, DMSO) δ ppm 7.36-7.97 (m, 6H), 13.19 (s, 1H).
References:
US2008/269110,2008,A1 Location in patent:Page/Page column 18
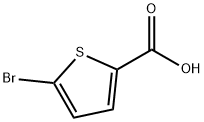
7311-63-9
343 suppliers
$5.00/250mg
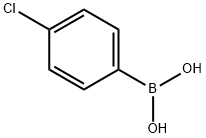
1679-18-1
618 suppliers
$10.00/1g

40133-14-0
32 suppliers
$42.40/1mg:

124-38-9
130 suppliers
$214.00/14L

40133-23-1
36 suppliers
$671.96/1g

40133-14-0
32 suppliers
$42.40/1mg:
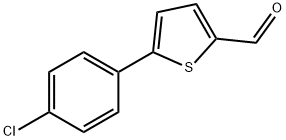
38401-71-7
62 suppliers
$174.00/1g

40133-14-0
32 suppliers
$42.40/1mg:

116016-54-7
0 suppliers
inquiry

40133-14-0
32 suppliers
$42.40/1mg: