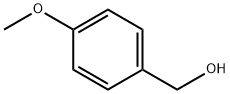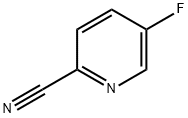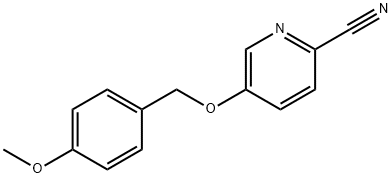
5-((4-Methoxybenzyl)oxy)picolinonitrile synthesis
- Product Name:5-((4-Methoxybenzyl)oxy)picolinonitrile
- CAS Number:917910-75-9
- Molecular formula:C14H12N2O2
- Molecular Weight:240.26
Yield:917910-75-9 60%
Reaction Conditions:
Stage #1: 4-Methoxybenzyl alcoholwith sodium hydride in N,N-dimethyl-formamide at 20; for 0.5 h;Cooling with ice;Inert atmosphere;
Stage #2: 2-Cyano-5-bromopyridine in N,N-dimethyl-formamide at 20; for 0.5 h;
Steps:
3.a
(a) 2-Cyano-5-(4-methoxybenzyloxy)pyridine; Sodium hydride (1.44 g, 33 mmol) was dissolved in DMF (20 mL), and 4-methoxybenzyl alcohol (3.74 mL, 33 mmol) was then added to the solution under a nitrogen atmosphere and under ice cooling. The obtained mixture was stirred at room temperature for 30 minutes. Thereafter, 5-bromo-2-cyanopyridine (4.58 g, 25 mmol) was added to the reaction solution, and the obtained mixture was further stirred at room temperature for 30 minutes. Thereafter, water was added to the reaction solution, and it was then extracted with ethyl acetate and washed with water. The water layers were combined and extracted with methylene chloride. The organic layers were combined, were dried over sodium sulfate, and were then concentrated. The obtained residue was recrystallized from ethyl acetate, so as to obtain the title compound (3.57 g, yield: 60%) in the form of yellow crystals.
References:
EP2308838,2011,A1 Location in patent:Page/Page column 56
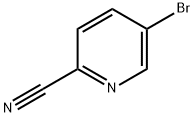
97483-77-7
588 suppliers
$10.00/5g

917910-75-9
12 suppliers
inquiry