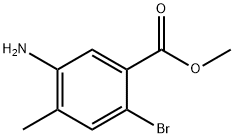
5-aMino-2-broMo-4-Methylbenzoic acid Methyl ester synthesis
- Product Name:5-aMino-2-broMo-4-Methylbenzoic acid Methyl ester
- CAS Number:474330-54-6
- Molecular formula:C9H10BrNO2
- Molecular Weight:244.09

67-56-1
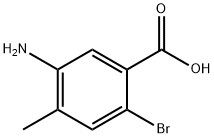
745048-63-9

474330-54-6
General procedure for the synthesis of methyl 5-amino-2-bromo-4-methylbenzoate from methanol and 5-amino-2-bromo-4-methylbenzoic acid: 5-amino-2-bromo-4-methylbenzoic acid (39.90 g, 0.17 mol) was suspended in methanol (500 mL). The suspension was cooled to 0 to 5 °C and thionyl chloride (40 mL, 0.54 mol) was added slowly and dropwise. The reaction mixture was heated to reflux for 6 hours. Upon completion of the reaction, the solution was concentrated under reduced pressure and the residue was diluted with ice water (500 g). The pH was adjusted to 7.5 with saturated aqueous sodium bicarbonate and subsequently extracted with ethyl acetate (3 x 200 mL). The organic phases were combined, washed with saturated saline (3 x 200 mL), dried over anhydrous magnesium sulfate, filtered and concentrated to dryness under reduced pressure to afford methyl 5-amino-2-bromo-4-methylbenzoate as a white solid (40.35 g, 95% yield).
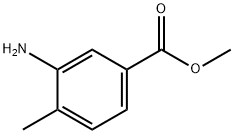
18595-18-1
366 suppliers
$8.00/10g

474330-54-6
72 suppliers
$62.77/0.5g
Yield: 90%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 0; for 0.333333 h;
Steps:
4.1 Step 1: Preparation of methyl 5-amino-2-bromo-4-methylbenzoate (4-b)
Dissolve methyl 3-amino-4-methylbenzoate (5g, 30.27mmol) in DMF (50mL), cool the system to 0 ° C, add NBS (5.39g, 30.27mmol) in portions, and stir at this temperature 20 minutes, TLC detection, the reaction was completed, the reaction solution was dropped into water, extracted with ethyl acetate, washed with water, dried, concentrated, and the residue was purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate = 2 / 1) The title compound (6.65 g, yield: 90.0%) was obtained in this step.
References:
Sichuan Kelun Botai Bio-pharmaceutical Co., Ltd.;Liu Jinming;Cai Jiaqiang;Tang Jianchuan;Wang Kunjian;Wang Lichun;Wang Jingyi CN110655503, 2020, A Location in patent:Paragraph 0254; 0255; 0256-0257

67-56-1
790 suppliers
$7.29/5ml-f

745048-63-9
62 suppliers
$45.00/25mg

474330-54-6
72 suppliers
$62.77/0.5g

745048-63-9
62 suppliers
$45.00/25mg

474330-54-6
72 suppliers
$62.77/0.5g
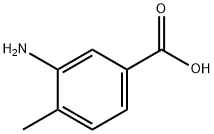
2458-12-0
350 suppliers
$15.00/5 g

474330-54-6
72 suppliers
$62.77/0.5g