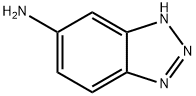
5-AMINOBENZOTRIAZOLE synthesis
- Product Name:5-AMINOBENZOTRIAZOLE
- CAS Number:3325-11-9
- Molecular formula:C6H6N4
- Molecular Weight:134.14
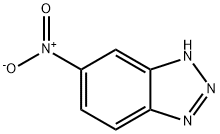
2338-12-7

3325-11-9
General procedure for the synthesis of 1H-benzo[d][1,2,3]triazol-5-amine from 5-nitrobenzotriazole: a mixture of 5-nitrobenzotriazole (10 mmol) and SnCl2-2H2O (50 mmol) in EtOAc (50 mL) was refluxed for 12 hours. The progress of the reaction was monitored by TLC and after confirming the completion of the reaction, the reaction mixture was cooled to room temperature. Subsequently, the pH of the reaction mixture was adjusted to 8-9 with saturated aqueous NaHCO3. The reaction mixture was extracted with EtOAc (2 × 150 mL) and the organic phases were combined. The organic phase was washed with brine (200 mL), dried over anhydrous Na2SO4, concentrated and purified by silica gel column chromatography (200-300 mesh) to afford 1H-benzo[d][1,2,3]triazol-5-amine. Yield: 90% as an orange solid, Melting point: 157-159°C (literature value not reported); IR (KBr, cm-1): 3375, 3077, 1630, 1521, 1430, 1320, 885, 842, 739; 1H NMR (500 MHz, DMSO-d6) δ: 7.65 (d, J = 8.5 Hz, 1H) , 6.73 (d, J = 8.0 Hz, 1H), 6.61 (s, 1H); HRMS (ESI): C6H7N4 ([M + H]+) Calculated value: 135.0665, measured value: 135.0665.
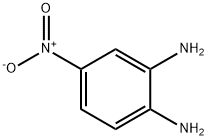
99-56-9
402 suppliers
$6.00/5g

3325-11-9
78 suppliers
$22.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: acetic acid; sodium nitrite / water / 8 h / 0 - 50 °C
2: tin(II) chloride dihdyrate / ethyl acetate / 12 h / Reflux
References:
Lv, Min;Ma, Jingchun;Li, Qin;Xu, Hui [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 2,p. 181 - 187]

2338-12-7
3 suppliers
$121.00/1g

3325-11-9
78 suppliers
$22.00/250mg