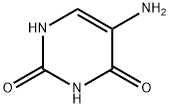
5-Aminouracil synthesis
- Product Name:5-Aminouracil
- CAS Number:932-52-5
- Molecular formula:C4H5N3O2
- Molecular Weight:127.1
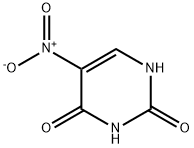
611-08-5

932-52-5
The general procedure for the synthesis of 5-aminouracil from 5-nitropyrimidine-2,4(1H,3H)-dione is as follows: 1. to a 5 liter four-necked round-bottomed flask was added water (2.871 L), ammonia (116.1 mL) and 5-nitropyrimidine-2,4(1H,3H)-dione (180 g, 1.15 mol, 1.00 equiv.). 2. sodium conidiosulfite (Na2S2O2, 860 g, 6.06 mol, 4.30 eq.) was added in batches. 3. The pH of the reaction solution was adjusted to 8 using 25% ammonia. 4. The reaction mixture was stirred at 75 °C for 3 hours. 5. After completion of the reaction, the mixture was cooled to 15 °C using an ice/water bath. 6. The solid product was collected by filtration to afford 5-aminopyrimidine-2,4(1H,3H)-dione (118 g, 81% yield) as a yellow solid.

611-08-5
383 suppliers
$5.00/5g

932-52-5
215 suppliers
$33.92/5g
Yield:932-52-5 92%
Reaction Conditions:
with Sodium hydrogenocarbonate;Na2S2O3 in lithium hydroxide monohydrate at 75; for 4 h;Reagent/catalyst;Temperature;
Steps:
11.1; 12.1; 13.1 step 1:
50 g of Na 2S 2 O 4 were added in portions to a saturated aqueous solution of 10 g of 5-nitrouracil and 20 g of sodium bicarbonate and stirred at 75° C. for 4 hours. The resulting white precipitate was filtered, washed with water and dried to obtain 7.4 g of 5-aminouracil (yield: 92%).
References:
WO2022/96015,2022,A1 Location in patent:Page/Page column 24-26
51-20-7
454 suppliers
$10.00/1g

932-52-5
215 suppliers
$33.92/5g

611-08-5
383 suppliers
$5.00/5g
20636-41-3
44 suppliers
$40.00/100mg

932-52-5
215 suppliers
$33.92/5g

23899-73-2
14 suppliers
inquiry

932-52-5
215 suppliers
$33.92/5g
7164-43-4
172 suppliers
$45.00/250mg

932-52-5
215 suppliers
$33.92/5g