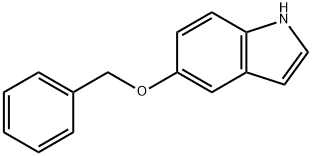
5-Benzyloxyindole synthesis
- Product Name:5-Benzyloxyindole
- CAS Number:1215-59-4
- Molecular formula:C15H13NO
- Molecular Weight:223.27
![1-[2-(5-BENZYLOXY-2-NITROPHENYL)VINYL]PYRROLIDINE](/StructureFile/ChemBookStructure7/GIF/CB1712320.gif)
153805-85-7
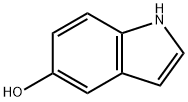
1953-54-4

1215-59-4
The reduction reaction of 4-benzyloxy-2-(2-pyrrolidinylvinyl)nitrobenzene was carried out using (E)-1-(5-(benzyloxy)-2-nitrophenylvinyl)pyrrolidine as a starting material, with reference to the method of Example 32, in the presence of 5% rhodium/carbon powder and a metal compound such as ferrous acetate (II), nickel nitrate (II) or cobalt acetylacetonate (III) as a catalyst. The results of the experiments are listed in Table 5 and show that in the presence of the reducing agent of the present invention, the yield of 5-benzyloxyindole was significantly increased while the yield of 5-hydroxyindole as a by-product was decreased as compared to the counterpart 4.
Yield:1215-59-4 99% ,1953-54-4 1%
Reaction Conditions:
with hydrogen;5% rhodium-on-charcoal;iron(II) acetate in tetrahydrofuran at 20; for 9 h;
Steps:
39
Following a procedure similar to Example 32, 4-benzyloxy-2-(2-pyrrolidinylvinyl)nitrobenzene as a starting material was reduced in the presence of 5 % rhodium/carbon powder and as a metal compound, ferrous(II) acetate, nickel(II) nitrate or cobalt(III) acetylacetonate as the catalyst of the present invention. The results are shown in the following Table 5, and in the case where the reducing agent of the present invention was used, 5-benzyloxyindole was obtained in a higher yield and 5-hydroxyindole was byproduced in a lower yield, compared to Comparison Example 4.
References:
EP1541582,2005,A1 Location in patent:Page/Page column 39

1953-54-4
382 suppliers
$12.00/1g

100-39-0
429 suppliers
$10.00/10g

1215-59-4
335 suppliers
$10.00/1g
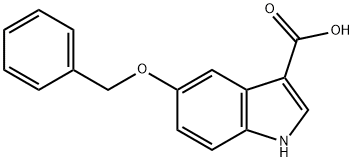
24370-73-8
13 suppliers
inquiry

1215-59-4
335 suppliers
$10.00/1g
![1-[2-(5-BENZYLOXY-2-NITROPHENYL)VINYL]PYRROLIDINE](/StructureFile/ChemBookStructure7/GIF/CB1712320.gif)
153805-85-7
8 suppliers
inquiry

1215-59-4
335 suppliers
$10.00/1g
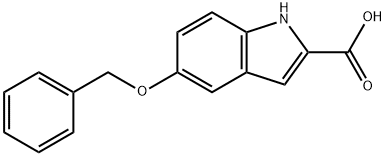
6640-09-1
108 suppliers
$21.00/250mg

1215-59-4
335 suppliers
$10.00/1g