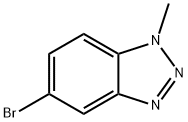
5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole synthesis
- Product Name:5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole
- CAS Number:944718-31-4
- Molecular formula:C7H6BrN3
- Molecular Weight:212.05
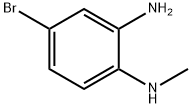
69038-76-2
![5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole](/CAS/20150408/GIF/944718-31-4.gif)
944718-31-4
General procedure for the synthesis of 5-bromo-1-methyl-1H-benzo[d][1,2,3]triazole from 4-bromo-N1-methylbenzene-1,2-diamine: 5-bromo-1-methyl-7H-benzo[2 (1.52 g, 22 mmol) was dissolved in water (5 ml) at 0 °C. The resulting solution was stirred at 0-10°C for 4 h. The pH was adjusted to 8 with potassium hydroxide (3N) and extracted with dichloromethane (2 x 200 ml). The organic layers were combined and concentrated in vacuum to give the residue. The residue was purified by silica gel chromatography (eluent: petroleum ether solution of 50% dichloromethane) to afford 5-bromo-1-methyl-1H-benzo[d][1,2,3]triazole as a red solid (1.5 g, 31% yield).LC/MS (ESI, m/z): [M + H]+ 213.1.1.1H-NMR (300 MHz, CDCl3) δ 8.24-8.25 (m, 1H), 7.60-7.63 (m, 1H), 7.42-7.46 (m, 1H), 4.32 (d, J = 5.4 Hz, 3H).

69038-76-2
75 suppliers
$28.00/100mg
![5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole](/CAS/20150408/GIF/944718-31-4.gif)
944718-31-4
61 suppliers
$45.00/10mg
Yield:944718-31-4 31%
Reaction Conditions:
Stage #1: 2-N-methylamino-5-bromoanilinewith hydrogenchloride;sodium nitrite in water at 0 - 10; for 4 h;
Stage #2: with potassium hydroxide in water; pH=8;
Steps:
95.3
Ste 3. 5-Bromo-l-methyl-7H-benzo[
References:
WO2012/119046,2012,A2 Location in patent:Page/Page column 207-208

74-88-4
356 suppliers
$15.00/10g

191230-40-7
6 suppliers
inquiry
![6-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole](/CAS/20150408/GIF/1083181-43-4.gif)
1083181-43-4
53 suppliers
$27.00/100mg
![5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole](/CAS/20150408/GIF/944718-31-4.gif)
944718-31-4
61 suppliers
$45.00/10mg
32046-62-1
113 suppliers
$9.00/250mg

64-19-7
1627 suppliers
$10.00/25ML
![5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole](/CAS/20150408/GIF/944718-31-4.gif)
944718-31-4
61 suppliers
$45.00/10mg
364-73-8
276 suppliers
$8.00/10g
![5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole](/CAS/20150408/GIF/944718-31-4.gif)
944718-31-4
61 suppliers
$45.00/10mg
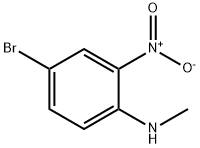
53484-26-7
86 suppliers
$30.00/250mg
![5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole](/CAS/20150408/GIF/944718-31-4.gif)
944718-31-4
61 suppliers
$45.00/10mg