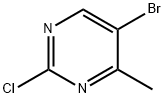
5-Bromo-2-chloro-4-methylpyrimidine synthesis
- Product Name:5-Bromo-2-chloro-4-methylpyrimidine
- CAS Number:633328-95-7
- Molecular formula:C5H4BrClN2
- Molecular Weight:207.46
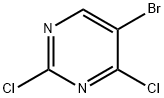
36082-50-5

75-16-1

633328-95-7
General procedure for the synthesis of 5-bromo-2-chloro-4-methylpyrimidine from 5-bromo-2,4-dichloropyrimidine and methylmagnesium bromide: A mixture of 5-bromo-2,4-dichloropyrimidine (0.4 g) and iron(III) acetylacetonate (0.062 g) in tetrahydrofuran (10 mL) was cooled to 0 °C. Methylmagnesium bromide (3M solution in diethyl ether, 0.76 mL) was added dropwise and the mixture was stirred at 0°C for 2 hours. Upon completion of the reaction, the reaction was quenched by the addition of saturated ammonium chloride solution, followed by extraction of the mixture with ethyl acetate (2 x 20 mL). The organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography using gradient elution with ethyl acetate and n-heptane to afford the target compound 5-bromo-2-chloro-4-methylpyrimidine (0.220 g, 60% yield) as a white solid.1H NMR (300 MHz, CDCl3) δ 8.52 (s,1H), 2.57 (s,3H).

36082-50-5
489 suppliers
$5.00/1g

75-16-1
274 suppliers
$12.00/10ml

633328-95-7
102 suppliers
$12.00/250mg
Yield: 60%
Reaction Conditions:
with iron(III)-acetylacetonate in tetrahydrofuran;diethyl ether at 0; for 25 h;
Steps:
16.1 Step I: 5-bromo-2-chloro-4-methyl-pyrimidine
A mixture of 5-Bromo 2,4-dichloropyrimidine (0.4 g) and lron(lll)acetylacetonate (0.062 g) in Tetrahydrofuran (10 mL) was cooled to 0 °C. Methyl magnessium bromide (3 M solution in Diethylether) (0.76 mL) was added dropwise and the mixture stirred for 2 h. Saturated Aminonium Chloride solution was added and the mixture extracted with Ethyl acetate (2 X 20 mL). The organic extracts were dried over anhydrous Sodium sulphate and evaporated under reduced pressure to get a residue which was purified by Silica gel flash column chromatography eluting with a gradient of Ethyl acetate and n-heptane to afford the desired product (0.220 g, 60 %) as a white solid. I H NMR (300 MHz, CDCI3) 6 8.52 (5, 1 H), 2.57 (5, 3H).
References:
BASF SE;SAMBASIVAN, Sunderraman;NARINE, Arun;CHAUDHURI, Rupsha;VALLINAYAGAM, Ramakrishnan;VYAS, Devendra;ADISECHAN, Ashokkumar;DATTA, Gopal Krishna WO2018/108671, 2018, A1 Location in patent:Page/Page column 169; 170
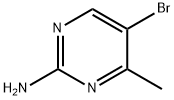
17321-93-6
148 suppliers
$8.00/250mg

633328-95-7
102 suppliers
$12.00/250mg