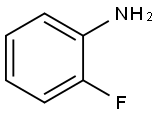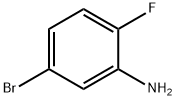
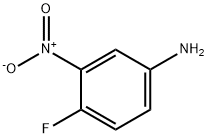
5-BROMO-2-FLUOROANILINE synthesis
- Product Name:5-BROMO-2-FLUOROANILINE
- CAS Number:2924-09-6
- Molecular formula:C6H5BrFN
- Molecular Weight:190.01
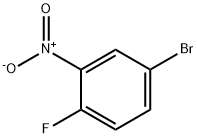
364-73-8

2924-09-6
General procedure for the synthesis of 5-bromo-2-fluoroaniline from 4-bromo-1-fluoro-2-nitrobenzene: 5-bromo-2-fluoro nitrobenzene (698 g) was dissolved in 95% ethanol (0.90 L), which was subsequently added to a mixed system of powdered iron (711 g) and saturated aqueous ammonium chloride (2.0 L). The reaction mixture was stirred continuously at 70°C for 24 hours (the reaction process was monitored by HPLC until complete). Upon completion of the reaction, the mixture was cooled to room temperature, filtered through diatomaceous earth and the filtrate was concentrated under reduced pressure to remove the solvent. The residue was subjected to liquid-liquid extraction with ethyl acetate (2 L) and water (2 L) to separate the organic and aqueous phases. The aqueous phase was further extracted once with ethyl acetate (1L). All organic phases were combined, washed with water (1 L), dried with anhydrous magnesium sulfate, filtered and the organic phase was concentrated under reduced pressure. Finally, 545.76 g of 5-bromo-2-fluoroaniline was obtained in 91% yield by high vacuum treatment for 5 h to completely remove the residual ethyl acetate.

364-73-8
275 suppliers
$8.00/10g

2924-09-6
207 suppliers
$5.00/1g
Yield:2924-09-6 91%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 70; for 24 h;
Steps:
5
Example 5; Preparation of 5-Bromo-2-fluoro-aniline; 5-Bromo-2-fluoro-nitrobenzene (698 g) is dissolved in 95% ethanol (0.90 L) and is added to a mixture of iron powder (711 g) in a saturated aqueous ammonium chloride solution (2.0 L). The reaction mixture is agitated for 24 hours at 7O0C (until the reaction is complete by HPLC). The reaction mixture is then cooled to room temperature, filtered through Celite, and the filtrate is evaporated under reduced pressure. The residue is extracted with ethyl acetate (2 L) and water (2 L) and the layers separated. The aqueous layer is extracted with ethyl acetate (1 L). The combined organic extracts are washed with water (1 L), dried over MgSO4, filtered, and the solution is concentrated under reduced pressure. The residual ethyl acetate is removed from the product under high vacuum for five hours to provide 545.76 g of 5- bromo-2-fluoro-aniline (91 % yield).
References:
PFIZER INC. WO2006/48761, 2006, A2 Location in patent:Page/Page column 32
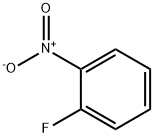
1493-27-2
510 suppliers
$5.00/10g

2924-09-6
207 suppliers
$5.00/1g
364-76-1
284 suppliers
$7.00/10g

2924-09-6
207 suppliers
$5.00/1g