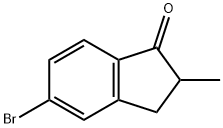
5-Bromo-2-methyl-1-indanone synthesis
- Product Name:5-Bromo-2-methyl-1-indanone
- CAS Number:104107-22-4
- Molecular formula:C10H9BrO
- Molecular Weight:225.08
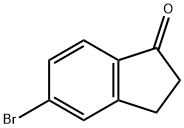
34598-49-7
385 suppliers
$14.00/5g

104107-22-4
33 suppliers
$156.00/50mg
Yield: 13%
Reaction Conditions:
with potassium tert-butylate in tetrahydrofuran;water
Steps:
5.a Preparation of (Z) and (E) 5-[1-[2-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl)naphthalenyl-methylene]-1,2,3,4-tetrahydronaphthalenyl]-1H-tetrazoles (CB02305 and CB58248)
a) 2-methyl-5-bromo-1-indanone. A solution of 5-bromo-1-indanone (3.75 g, 17.76 mmol) in 18 ml of anhydrous THF is brought to -20° C. under an atmosphere of argon and with magnetic stirring. A 1M solution of t-BuOK in THF (18 ml, 18 mmol) is added with a syringe and the stirring continued for 3 hours at ambient temperature. At 0° C., 10 ml of distilled water is added and the mixture then extracted with ether (4*75 ml), dried over MgSO4, filtered and evaporated to obtain a raw product that is incorporated onto silica and purified by flash chromatography on silica (eluent ether:petroleum ether=2:98 up to 5:95). In order of elution, one obtains 1.85 g of a yellowish oil, 2,2-dimethyl-5-bromo-1-indanone (yield=43%), 0.50 g of a white solid 2-methyl-5-bromo-1-indanone (yield=13%) and 1.20 g of a white solid (32%) the starting material, 5-bromo-1-indanone. NMR1H 200 MHz (CDCl3): 1.25 (d, 3H, Me J 7 Hz); 2.55-2.75 (m, 2H, -CH2-); 3.25-3.45 (m, 1H, -CHMe-); 7.40-7.65 (m, 3H, ArH).
References:
Centre Europeen de Bioprospective-CEB US6239284, 2001, B1
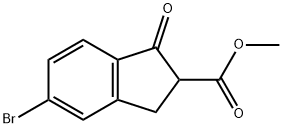
628732-08-1
3 suppliers
inquiry

104107-22-4
33 suppliers
$156.00/50mg

50-00-0
896 suppliers
$10.00/25g
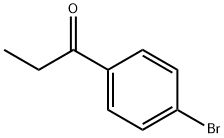
10342-83-3
225 suppliers
$5.00/5g

104107-22-4
33 suppliers
$156.00/50mg