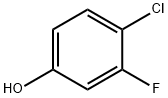
5-CHLORO-4-FLUORO-2-HYDROXY-BENZALDEHYDE synthesis
- Product Name:5-CHLORO-4-FLUORO-2-HYDROXY-BENZALDEHYDE
- CAS Number:264879-16-5
- Molecular formula:C7H4ClFO2
- Molecular Weight:174.56
Yield:264879-16-5 60.6%
Reaction Conditions:
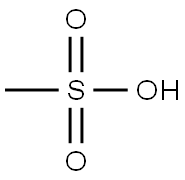
Stage #1: methanesulfonic acid;4-chloro-3-fluorophenol at 20;
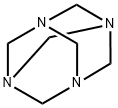
Stage #2: with hexamethylenetetramine at 10 - 100;
Steps:
7.a.1 Example 7a; 6-CHLORO-7-(ETHYLTHIO)-2-(TRIFLUOROMETHYL)-2H-CHROMENE-3-CARBOXYLIC acid; Step 1. Preparation of 5-CHLORO-4-FLUORO-2-HYDROXYBENZALDEHYDE.
[0208] To 4-chloro-3-fluorophenol (25 g, 171 mmole) was added the methanesulfonic acid (130 mL) and the mixture was stirred at r. t. An ice-water bath was used to bring the temperature of the stirred mixture to 10 °C. Methenamine (47. 8 g, 341 mmole) was added portionwise in 3 gm scoops to allow the solid to dissolve and keep the temperature below 40 °C. Addition was complete after 90 minutes. -CAUTION : If the addition is carried out too fast, the solid will react exothermically with the acid and decompose. The mixture was heated to 100 °C. At 70 °C, a change in the reaction mixture color was noticed and a solid formed. Once the temperature of 100 °C was reached, the heating manifold was removed and the mixture allowed to cool to r. t. The reaction mixture was poured into 1L of ice water and extracted 3x W/CH2CL2. The combined extracts were filtered through a silica plug (4.5 x 9 cm), washed with additional CHUCK and concd to give a crude yellow solid. Kugelrohr distillation (100 millitorr, 60 °C) gave 18.06 g (60.6%) of a white solid : 1HNMR shows >95% purity : IH NMR (CDCL3) 6.79 (d, 1H, J= 10. 3 Hz), 7.62 (d, 1H, J=7. 9 Hz), 9.80 (s, 1H), 11.23 (d, 1H, J=1. 5 HZ).Preparation of Ethyl 6-CHLORO-7-FLUORO-2- (TRIFLUOROMETHYL)-2H-CHROMENE-3- carboxylate COVET 0 hexamethylene- tetramine 3C OEt > i'K CO, DMF F 3SO3H FaOCF3 Step 1. Preparation of 5-CHLORO-4-FLUORO-2-HYDROXYBENZALDEHYDE. [0766] To the 4-chloro-3-fluorophenol (25 g, 171 mmole) was added methanesulfonic acid (130 mL) and the mixture was stirred at rt. An ice-water bath was used to bring the temperature of the stirred mixture to 10 °C. METHENAMINE (47.8 g, 341 mmole) was added portionwise in 3 gm scoops to allow the solid to dissolve and keep the temperature below 40 °C. Addition was complete after 90 minutes. -CAUTION : If the addition is carried out too fast, the solid will react exothermically with the acid and decompose. The mixture was heated to 100 °C. At 70 °C, a change in the reaction mixture color was noticed and a solid formed. Once the temperature of 100 °C was reached, the heating manifold was removed and the mixture allowed to cool to rt. The reaction mixture was poured into 1L of ice water and extracted 3 times with CH2C12. The combined extracts were filtered through a silica plug (4.5 x 9 cm), washed with additional CH2C12 and concd to give a crude yellow solid. Kugelrohr distillation (100 mtorr, 60 °C) gave 18.06 g (60.6%) of a white solid : 1H NMR (CDCl3) 6.79 (d, 1H, J= 10. 3 Hz), 7.62 (d, 1H, J=7. 9 Hz), 9.80 (s, 1H), 11.23 (d, 1H, J=1. 5 Hz).
References:
WO2004/87686,2004,A2 Location in patent:Page 117-118; 364-365 WO2004/87687,2004,A1 Location in patent:Page 117-118; 364-365
348-60-7
194 suppliers
$8.00/1g

264879-16-5
26 suppliers
inquiry