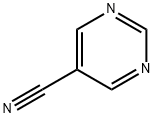
5-CYANOPYRIMIDINE synthesis
- Product Name:5-CYANOPYRIMIDINE
- CAS Number:40805-79-6
- Molecular formula:C5H3N3
- Molecular Weight:105.1
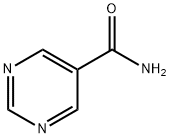
40929-49-5

40805-79-6
General procedure for the synthesis of 5-cyanopyrimidine from pyrimidine-5-carboxamide: To a suspension of anhydrous dichloromethane (15 mL) containing pyrimidine-5-carboxamide (262 mg, 2.12 mmol) and triethylamine (481 mg, 4.24 mmol) was added slowly and dropwise a solution of trifluoroacetic anhydride (0.36 mL, dissolved in 4 mL of dichloromethane) at 0 °C. The reaction mixture was stirred at 0°C to room temperature for 2 hours. After completion of the reaction, it was quenched with water (2 mL) and the organic layer was washed sequentially with 1N NaOH solution (5 mL) and brine (2 x 5 mL). The organic phase was dried with anhydrous magnesium sulfate and subsequently concentrated under reduced pressure at less than 30 °C to afford the light yellow solid product 5-cyanopyrimidine (174 mg, 78% yield). The product was characterized by 1H NMR (400 MHz, DMSO): δ 9.44 (s, 1H), 9.31 (s, 2H).

40929-49-5
34 suppliers
inquiry

40805-79-6
140 suppliers
inquiry
Yield: 78%
Reaction Conditions:
Stage #1:pyrimidine-5-carboxamide with triethylamine;trifluoroacetic anhydride in dichloromethane at 0 - 20;
Stage #2: with sodium hydroxide in dichloromethane;water
Steps:
5
Pyrimidine-5-carbonitrile (SO3-067): To a suspension of pyrimidine-5-carboxamide (SOS- OSS) (262 mg, 2.12 mmol) and triethyl amine (481 mg, 4.24 mmol) in anhydrous dichoromethane (15 ml) was slowly added a solutionf of trifuoroacetic anhydride (0.36 ml in 4 ml dichloromethane) at 0°C. The reaction mixture was stirred at 0°C to RT for 2 h. and quenched with water (2ml) and washed with NaOH (1 N, 5 ml) and brine (2 x 5 ml). Organic solvent was dried (MgS04) and evaporated at less than 30°C to provide SO3-067 as a pale yellow solid. (1 74 mg, 78%). 1 H NM R (400 M Hz, DMSO) δ 9.44 (s, 1 H), 9.31 (s, 2H).
References:
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC.;LAWRENCE, Harshani R.;SEBTI, Said M.;OZCAN, Sevil WO2012/129564, 2012, A2 Location in patent:Page/Page column 79
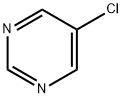
17180-94-8
139 suppliers
$27.00/1g
557-21-1
106 suppliers
$12.00/5g

40805-79-6
140 suppliers
inquiry
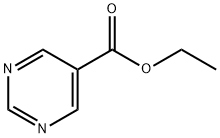
40929-50-8
83 suppliers
$15.00/1g

40805-79-6
140 suppliers
inquiry

75-05-8
1049 suppliers
$10.00/10g
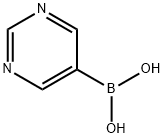
109299-78-7
302 suppliers
$10.00/1g

40805-79-6
140 suppliers
inquiry