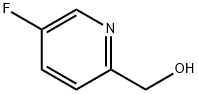
5-FLUORO-2-HYDROXYMETHYL PYRIDINE synthesis
- Product Name:5-FLUORO-2-HYDROXYMETHYL PYRIDINE
- CAS Number:802325-29-7
- Molecular formula:C6H6FNO
- Molecular Weight:127.12
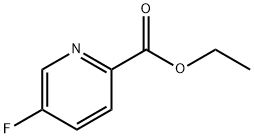
148541-70-2

802325-29-7
General procedure for the synthesis of (5-fluoropyridin-2-yl)methanol from ethyl 5-fluoropyridine-2-carboxylate: to a solution of ethyl 5-fluoropyridine-2-carboxylate (377 mg, 2.23 mmol) in tetrahydrofuran (6 mL) was slowly added lithium aluminum hydride (LiAlH4, 73 mg, 3.34 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 1 hour. Upon completion of the reaction, it was quenched with ice water and the organic layer was subsequently extracted with ethyl acetate. The organic layer was dried with anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography with ethyl acetate/hexane (20:80 to 60:40) as eluent to give (5-fluoropyridin-2-yl)methanol (309 mg, 54% yield, colorless oil).
107504-08-5
278 suppliers
$7.00/250mg

802325-29-7
104 suppliers
$50.00/250mg
Yield: 52%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 12 h;Inert atmosphere;
Steps:
67
A mixture of 14 (500 mg, 4 mmol) and LiAIH4(202 mg, 5 mmol) in THF (10 mL) was degassed and purged with N2for 3 times, and then the mixture was stirred at 0 °C for 12 h under N2atmosphere. The reaction mixture was quenched by saturated sodium potassium tartrate (0.8 mL) at 15 °C, and then filtered. The mixture was diluted with H20 (5 mL) and extracted with EtOAc (5 mL x 3). The combined organic layer was washed with brine (5 mL x 2), dried over Na2S04, filtered and concentrated under reduced pressure to give 15 (236 mg, 52%) as a yellow solid.1H NMR (400 MHz, DMSO-d6) 8.43 (d, J - 2.8 Hz, 1 H), 7.45-7.41 (m, 1 H), 7.31-7.27 (m, 1 H), 4.76 (s, 2H). A mixture of 15 (1 g, 8 mmol), DCC (3 g, 16 mmol), DMAP (96 mg, 786 umol) and A/-BOC-(S)-valine (2 g, 9 mmol) in DCM (5 mL) was degassed and purged with N2for 3 times, and then the mixture was stirred at 25 "C for 12 h under N2atmosphere. The reaction mixture was filtered and concentrated under reduced pressure. The residue was purified by column chromatography (Si02, PE/EtOAc = 5:1) to give 16 (1 .3 g, 51 %) as a yellow liquid.1H N R (400 MHz, DMSO-de) 8.55 (s, 1 H), 7.71-7.83 (m, 1 H), 7.54-7.51 (m, 1 H), 7.25 (d, J = 8.0 Hz, 1 H), 5.22-5.13 (m, 2H), 3.91 (t, J = 7.2 Hz, 1 H), 2.06-2.02 (m, 1 H), 1.38 (s, 9H), 0.87 (d, J - 6.4 Hz, 6H).
References:
ANACOR PHARMACEUTICALS, INC.;AKAMA, Tsutomu;CARTER, David Scott;HALLADAY, Jason S.;JACOBS, Robert T.;LIU, Yang;PLATTNER, Jacob J.;ZHANG, Yong-Kang;WITTY, Michael John WO2017/195069, 2017, A1 Location in patent:Page/Page column 61
107504-07-4
131 suppliers
$24.00/250mg

802325-29-7
104 suppliers
$50.00/250mg

148541-70-2
35 suppliers
$116.64/250mgs:

802325-29-7
104 suppliers
$50.00/250mg
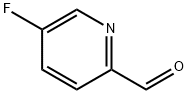
31181-88-1
169 suppliers
$11.00/250mg

802325-29-7
104 suppliers
$50.00/250mg