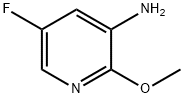
5-Fluoro-2-Methoxy-pyridin-3-ylaMine synthesis
- Product Name:5-Fluoro-2-Methoxy-pyridin-3-ylaMine
- CAS Number:1211541-93-3
- Molecular formula:C6H7FN2O
- Molecular Weight:142.13
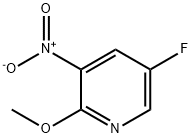
1211534-27-8

1211541-93-3
c) 5-fluoro-2-methoxy-3-nitropyridine (Preparation 5b, 0.300 g, 1.74 mmol) was used as a raw material, which was dissolved in ethanol and 10% palladium carbon catalyst (0.300 g) was added. The reaction mixture was stirred at room temperature and under hydrogen atmosphere. after 5 h, the reaction was completed and the catalyst was removed by Celite filtration. The filter cake was washed with ethanol and the filtrate and washings were combined and concentrated to afford the target product 5-fluoro-2-methoxypyridin-3-amine (0.220 g, 89% yield) as a brown solid. Low resolution mass spectrometry (LRMS) showed m/z: 143 (M+1)+. 1H NMR (300 MHz, CDCl3) data: δ 3.96 (s, 3H), 6.67 (dd, 1H), 7.39 (d, 1H).

1211534-27-8
31 suppliers
$45.00/10mg

1211541-93-3
51 suppliers
$56.00/100mg
Yield: 89%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol at 20; for 5 h;
Steps:
5.c
c) 5-Fluoro-2-methoxypyridin-3-amine 10% Palladium on carbon (0.300 g) was added to a solution of 5-fluoro-2-methoxy-3-nitropyridine (Preparation 5b, 0.300 g, 1.74 mmol) in ethanol (15 mL) and the reaction mixture was stirred at ambient temperature under a hydrogen atmosphere. After 5 hours, the mixture was then filtered through Celite and the filter cake was washed with ethanol. The combined filtrate and washings were concentrated to give the title compound (0.220 g, 89%) as a brown solid. LRMS (m/z): 143 (M+1)+. 1H NMR δ (300 MHz, CDCl3): 3.96 (s, 3H), 6.67 (dd, 1H), 7.39 (d, 1H).
References:
Almirall, S.A. EP2527344, 2012, A1 Location in patent:Page/Page column 22-23
136888-20-5
142 suppliers
$8.00/100mg

1211541-93-3
51 suppliers
$56.00/100mg
51173-05-8
215 suppliers
$6.00/250mg

1211541-93-3
51 suppliers
$56.00/100mg
84476-99-3
280 suppliers
$8.00/1g

1211541-93-3
51 suppliers
$56.00/100mg