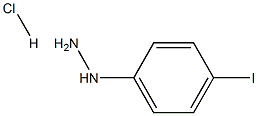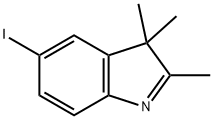
5-iodo-2,3,3-trimethyl-3H-indole synthesis
- Product Name:5-iodo-2,3,3-trimethyl-3H-indole
- CAS Number:54136-25-3
- Molecular formula:C11H12IN
- Molecular Weight:285.12
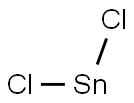
7772-99-8
446 suppliers
$10.00/25g
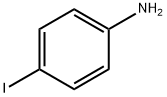
540-37-4
469 suppliers
$6.00/5g

54136-25-3
28 suppliers
inquiry
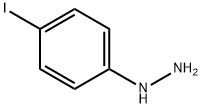
13116-27-3
121 suppliers
$15.00/100mg
Yield: 50%
Reaction Conditions:
with hydrogenchloride;sodium nitrate in (2S)-N-methyl-1-phenylpropan-2-amine hydrate;hexane;water;toluene
Steps:
3.g g.
Sodium nitrate (16.56 g, 0.24 mol, Aldrich) dissolved in water (100 ml) was added dropwise within 45 mins. to a solution of 4-iodoaniline (43.9 g, 0.20 mol, Aldrich) in ice-water (600 ml) and concentrated hydrochloric acid (200 ml) which was cooled to 0°-2° C. The reaction mixture was stirred for a further 30 minutess at 0°-2° C. then tin(II) chloride (151.68 g, 0.8 mol, Aldrich) in c.HCl (150 ml) was added dropwise over 90 mins. while maintaining the temp. of the reaction mixture between 0°-2° C. Following the addition, the resulting solution was warmed to room temperature and stirred for 3 hours. The yellow solid which had separated from the solution was then collected by filtration, placed in ice water (800 ml) and the pH adjusted to 10 with 25% aqueous potassium hydroxide solution. The resulting solid was collected by filtration, washed with a small amount of water and dried under vacuum. The product was then placed in toluene (400 ml) and filtered to remove insoluble impurities. Hexane (1200 ml) was added and upon cooling in the refrigerator yellow needles separated which were collected by filtration, washed with hexane (100 ml) and dried under high vacuum to give pure 4-Iodophenylhydrazine (23.36 g, 50%), m.p.=94° C. 5-Iodo-2,3,3-trimethyl-(3H)-indolenine (9) was prepared by a modification of the procedure of Moreau et al., Eur. J. Med. Chem. Chim. Ther., 9 (3): 274-280 (1974).
References:
Zynaxis, Inc. US5667764, 1997, A
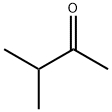
563-80-4
315 suppliers
$17.00/25mL

13116-27-3
121 suppliers
$15.00/100mg

54136-25-3
28 suppliers
inquiry

540-37-4
469 suppliers
$6.00/5g

54136-25-3
28 suppliers
inquiry