5-IODOTUBERCIDIN synthesis
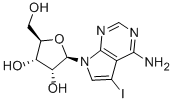
- Product Name:5-IODOTUBERCIDIN
- CAS Number:24386-93-4
- Molecular formula:C11H13IN4O4
- Molecular Weight:392.15
![7H-Pyrrolo[2,3-d]pyriMidine, 4-chloro-5-iodo-7-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)-](/CAS/20211123/GIF/480439-89-2.gif)
480439-89-2

24386-93-4
A mixture of (2R,3R,4R,5R)-2-((benzoyloxy)methyl)-5-(4-chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-7-yl)tetrahydrofuroyl-3,4-diyl dibenzoate (3.0 g, 4.1 mmol) was used as a raw material, and a mixture of it with aqueous ammonia solution (45 ml) and 1,4-dioxane (45 ml) was reacted at 80 °C The reaction was stirred for 16 hours. The completion of the reaction was confirmed by LC-MS analysis. The reaction mixture was concentrated to remove the solvent to give a residue. The residue was suspended in a solvent mixture of CH2Cl2/MeOH (9:1) and purified by passing through a column pre-filled with silica gel (using CH2Cl2/MeOH (9:1→1:1) as eluent). The eluate containing the target product was collected and concentrated to afford (2R,3R,4S,5R)-2-(4-amino-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (1.1 g, 69% yield) as a white solid. The product was characterized by 1H NMR (DMSO-d6): δ 8.10 (s, 1H), 7.68 (s, 1H), 6.68 (br s, 2H), 6.03 (d, 1H), 5.33 (br s, 1H), 5.15 (br, 2H), 4.36 (br s, 1H), 4.07 (br s, 1H), 3.88 (m, 1H). 3.56 (m, 2H).
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
840 suppliers
$6.00/1g

24386-93-4
173 suppliers
$13.00/100μg
Yield:-
Steps:
Multi-step reaction with 4 steps
1: N-iodosuccinimide / 20 h / 20 °C
2: NaH / 24 h / 20 °C
3: 100 percent / TFA
4: 88.7 percent / NH3 / methanol / 22 h / 116 °C
References:
Zhang, Liangren;Zhang, Yunlong;Li, Xianghui;Zhang, Lihe [Bioorganic and Medicinal Chemistry,2002,vol. 10,# 4,p. 907 - 912]
![7H-Pyrrolo[2,3-d]pyriMidine, 4-chloro-5-iodo-7-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)-](/CAS/20211123/GIF/480439-89-2.gif)
480439-89-2
16 suppliers
inquiry

24386-93-4
173 suppliers
$13.00/100μg
![7H-Pyrrolo[2,3-d]pyrimidine,4-chloro-5-iodo-7-b-D-ribofuranosyl-](/CAS/20180601/GIF/24386-91-2.gif)
24386-91-2
3 suppliers
inquiry

24386-93-4
173 suppliers
$13.00/100μg
![4-Chloro-5-iodo-7H-pyrrol[2,3-d]pyrimidine](/CAS/GIF/123148-78-7.gif)
123148-78-7
271 suppliers
$8.00/1g

24386-93-4
173 suppliers
$13.00/100μg
![α-D-Ribofuranosyl chloride, 5-O-[(1,1-dimethylethyl)dimethylsilyl]-2,3-O-(1-methylethylidene)-](/CAS/20210305/GIF/102690-94-8.gif)
102690-94-8
1 suppliers
inquiry

24386-93-4
173 suppliers
$13.00/100μg