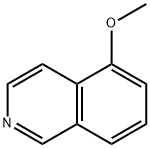
5-methoxyisoquinoline-1-carbonitrile synthesis
- Product Name:5-methoxyisoquinoline-1-carbonitrile
- CAS Number:90806-60-3
- Molecular formula:C11H8N2O
- Molecular Weight:184.19
Yield:90806-60-3 98%
Reaction Conditions:
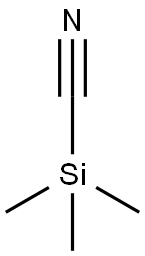
with triethylamine in acetonitrile at 20 - 75; for 20 h;Inert atmosphere;
Steps:
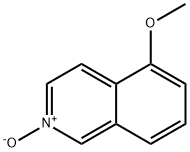
To a stirred solution of Cap 138, step b (0.70 g, 4.00 mmol) and triethylamine (1.1 mL, 8.00 mmol) in dry acetonitrile (20 mL) at room temperature under nitrogen was added trimethylsilylcyanide (1.60 mL, 12.00 mmol). The mixture was heated at 75 °C for 20 h before it was cooled to room temperature, diluted with ethyl acetate and washed with saturated sodium bicarbonate solution and brine prior to drying over a2S04 and solvent concentration. The residue was flash chromatographed on silica gel (gradient elution with 5% ethyl acetate in hexanes to 25% ethyl acetate in hexanes) to afford Cap-138, step c (498.7 mg, 68%) as a white, crystalline solid along with 223 mg (30%) of additional Cap-138, step c recovered from the filtrate. ? NMR (CDC13, 500 MHz) δ 8.63 (d, J= 5.5 Hz, 1H), 8.26 (d, J= 5.5 Hz, 1H), 7.88 (d, J= 8.5 Hz, 1H), 7.69 (t, J= 8.0 Hz, 1H), 7.08 (d, J= 7.5 Hz, 1H), 4.04 (s, 3H); Rt = 1.75 min, (Cond.-Dl); 90% homogeneity index; LCMS: Anal. Calc. for [M+H]+ C11H9N2O: 185.07; found: 185.10.
References:
WO2012/18325,2012,A1 Location in patent:Page/Page column 105-106
2439-04-5
160 suppliers
$9.00/250mg

90806-60-3
17 suppliers
inquiry
90806-58-9
70 suppliers
$16.00/100mg

90806-60-3
17 suppliers
inquiry