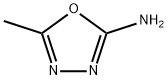
5-METHYL-1,3,4-OXADIAZOL-2-YLAMINE synthesis
- Product Name:5-METHYL-1,3,4-OXADIAZOL-2-YLAMINE
- CAS Number:52838-39-8
- Molecular formula:C3H5N3O
- Molecular Weight:99.09

2302-87-6

52838-39-8
General procedure for the synthesis of 5-methyl-1,3,4-oxadiazol-2-amine from 2-acetylhydrazinecarboxamide: 1.17 g (9.99 mmol) of 2-acetylhydrazinecarboxamide was suspended in 23 mL of dimethoxyethane, and 1.68 g (10.96 mmol) of phosphorochloride was added. The reaction mixture was stirred at 70 °C for 2 hours. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. To the residue, 10 mL of water and 100 mL of ethyl acetate were added, followed by the addition of sodium carbonate to adjust the pH of the aqueous phase to 8. The mixed solution was extracted with ethyl acetate (100 mL x 2), and the organic layers were combined and dried over anhydrous sodium sulfate. The organic solvent was removed by distillation under reduced pressure to give 0.50 g of 5-methyl-1,3,4-oxadiazol-2-amine as a white solid.

2302-87-6
6 suppliers
inquiry

52838-39-8
115 suppliers
$8.00/100mg
Yield: 0.50 g
Reaction Conditions:
with trichlorophosphate in 1,2-dimethoxyethane at 70; for 2 h;
Steps:
18.2 Synthesis of 2-amino-5-methyl-1,3,4-oxadiazole
In the case of2-Acetylhydrazinecarboxamide1.17 g (9.99 mmol)Was suspended in 23 ml of dimethoxyethane,1.68 g (10.96 mmol) of phosphorus oxychloride was added,And the mixture was stirred at 70 ° C. for 2 hours. After completion of stirring, the solvent in the reaction mixture was distilled off under reduced pressure, 10 ml of water and 100 ml of ethyl acetate were added, and sodium carbonate was added until the pH of the aqueous phase became 8. The resulting mixed solution was extracted with ethyl acetate (100 ml × twice), the organic layer was dried with sodium sulfate, and the solvent was distilled off to obtain 0.50 g of the objective compound as a white solid.
References:
NISSAN CHEMICAL INDUSTRIES LIMITED;NAKAYA, YOSHIHIKO;TANIMA, DAISUKE;INABA, MASAMITSU;MIYAKADO, YUUKI;FURUHASHI, TAKAMASA;MAEDA, KAZUSHIGE JP2017/25054, 2017, A Location in patent:Paragraph 0230

99319-29-6
0 suppliers
inquiry

52838-39-8
115 suppliers
$8.00/100mg
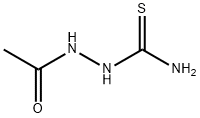
2302-88-7
83 suppliers
$11.00/1g

52838-39-8
115 suppliers
$8.00/100mg

2302-88-7
83 suppliers
$11.00/1g
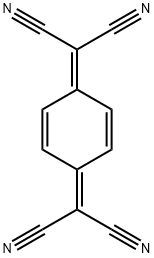
1518-16-7
199 suppliers
$11.00/250mg

52838-39-8
115 suppliers
$8.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
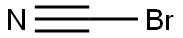
506-68-3
166 suppliers
$10.00/1g
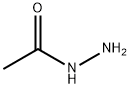
1068-57-1
417 suppliers
$6.00/5g

52838-39-8
115 suppliers
$8.00/100mg