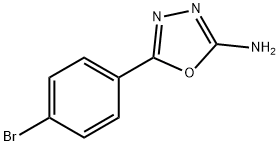
5-(4-BROMOPHENYL)-1,3,4-OXADIAZOL-2-AMINE synthesis
- Product Name:5-(4-BROMOPHENYL)-1,3,4-OXADIAZOL-2-AMINE
- CAS Number:33621-62-4
- Molecular formula:C8H6BrN3O
- Molecular Weight:240.06
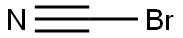
506-68-3
164 suppliers
$10.00/1g
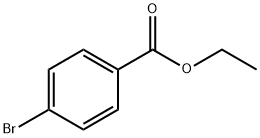
5798-75-4
204 suppliers
$6.00/5g

33621-62-4
19 suppliers
$125.00/1g
Yield:33621-62-4 72%
Reaction Conditions:
Stage #1: Ethyl 4-bromobenzoatewith hydrazine hydrate in ethanol; for 16 h;Reflux;
Stage #2: bromocyane in ethanol; for 16 h;Reflux;
Steps:
5-(4-bromophenyl)-1,3,4-oxadiazol-2-amine (1)
To a stirred solution of ethyl 4-bromobenzoate I (50 g, 0.218 mol) in Ethanol (750 mL) was added hydrazine hydrate (50 mL, 1 vol) and stirred at reflux temp for 16 h. The solvent was evaporated, the residue was triturated with pet ether to give hydrazide. The hydrazide was taken in Ethanol (500 mL) and added cyanogen bromide (34 g, 0.327 mmol) and stirred at RT for 16 h. After completion, the solvent was evaporated and the residue was poured into ice water and filtered the solid to obtain oxadiazole 1 (37.7 g, 72%, over two steps) as a white solid; m. p. 270-272 °C; IR (KBr) υ: 3292, 3110, 1660 cm-1; 1H NMR (DMSO-d6, 300 MHz): δ 7.75 (t, 4H, Phenyl), 7.35 (s, 2H, -NH2) 13CNMR (DMSO-d6, 75 MHz): δ 163.7, 156.6, 132.3, 126.9, 123.7, 123.4; Lit-35 1H NMR (CDCl3): δ 7.25-7.69 (dd, 4H, J = 2.6, 5.6 Hz, Ar-H), 7.75 (s, 2H, NH); 13C NMR (CDCl3): 144-130 (Ar-C).LC-MS: m/z 240.09 (M+H). HRMS. Calcd. for C8H7BrN3O: 240.0875, Found: 240.0879.
References:
Devarasetty, Kiran;Tharikoppula, Giri;Sridhar, Tailor;Eppakayala, Laxminarayana;Kyasani, Mahesh;Arumugam, Premkumar;Pusuluri, Srinivas [Synthetic Communications,2016,vol. 46,# 3,p. 263 - 274] Location in patent:supporting information
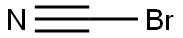
506-68-3
164 suppliers
$10.00/1g
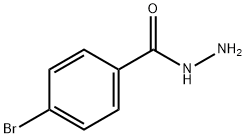
5933-32-4
168 suppliers
$8.00/1g

33621-62-4
19 suppliers
$125.00/1g
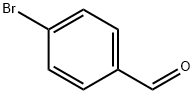
1122-91-4
701 suppliers
$9.00/10g

33621-62-4
19 suppliers
$125.00/1g
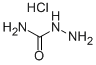
563-41-7
550 suppliers
$5.00/25g

1122-91-4
701 suppliers
$9.00/10g

33621-62-4
19 suppliers
$125.00/1g
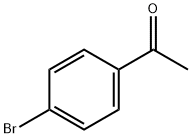
99-90-1
481 suppliers
$6.00/25g

33621-62-4
19 suppliers
$125.00/1g