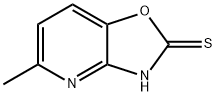
5-Methyl[1,3]oxazolo[4,5-b]pyridine-2-thiol synthesis
- Product Name:5-Methyl[1,3]oxazolo[4,5-b]pyridine-2-thiol
- CAS Number:55656-32-1
- Molecular formula:C7H6N2OS
- Molecular Weight:166.2
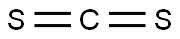
75-15-0
266 suppliers
$40.00/100g
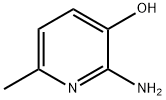
20348-16-7
96 suppliers
$42.00/100mg
![5-Methyl[1,3]oxazolo[4,5-b]pyridine-2-thiol](/CAS/20140525/GIF/CB72557033.gif)
55656-32-1
17 suppliers
$110.00/50mg
Yield:55656-32-1 85%
Reaction Conditions:
Stage #1: carbon disulfide;2-amino-3-hydroxy-6-methylpyridinewith potassium hydroxide in ethanol; for 16 h;Heating / reflux;
Stage #2: with acetic acid in water; pH=5;
Steps:
Methviri.31oxazolor4.5-bipyridine-2-thiol; KOH (14 g, 0.25 mol) was added to a stirred mixture of 2-amino-6-methylpyridin-3-ol (15.5 g, 0.125 mol) in absolute EtOH (500 mL) under an argon atmosphere. Carbon disulfide (16 mL, 0.25 mol) was added and the reaction mixture was heated to reflux in argon for 16 h. The reaction mixture was evaporated to dryness, water (250 mL) was added followed by acetic acid (20 mL) until the pH was ~5. The formed precipitate was separated by filtration, washed with water, and dried to afford 17.7 g (85%) of 5-methyl[1,3]oxazolo[4,5-b]pyridine-2- thiol as a yellow crystalline solid. 1H-NMR-data (DMSO-d6): 7.70 (1H), 7.07 (1H), 2.46 (3H).
References:
WO2006/51410,2006,A1 Location in patent:Page/Page column 39
140-89-6
306 suppliers
$12.00/5g

20348-16-7
96 suppliers
$42.00/100mg
![5-Methyl[1,3]oxazolo[4,5-b]pyridine-2-thiol](/CAS/20140525/GIF/CB72557033.gif)
55656-32-1
17 suppliers
$110.00/50mg
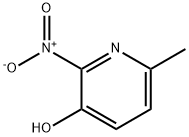
15128-90-2
136 suppliers
$30.00/5G
![5-Methyl[1,3]oxazolo[4,5-b]pyridine-2-thiol](/CAS/20140525/GIF/CB72557033.gif)
55656-32-1
17 suppliers
$110.00/50mg