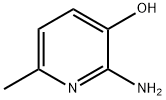
2-amino-6-methylpyridin-3-ol synthesis
- Product Name:2-amino-6-methylpyridin-3-ol
- CAS Number:20348-16-7
- Molecular formula:C6H8N2O
- Molecular Weight:124.14
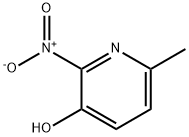
15128-90-2

20348-16-7
General procedure for the synthesis of 2-amino-3-hydroxy-6-methylpyridine from 3-hydroxy-6-methyl-2-nitropyridine: 3-hydroxy-6-methyl-2-nitropyridine (30 g, 194.6 mmol) was dissolved in ethanol and the hydrogenation reaction was carried out for 1 hr at 50 °C using hydrogen (5 psi) and 20% Pd(OH)2/C catalyst. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated under reduced pressure to afford the target product 2-amino-3-hydroxy-6-methylpyridine as a brown solid (23.68 g, 98% yield). The NMR data of the product was consistent with the expected structure.

15128-90-2
138 suppliers
$30.00/5G

20348-16-7
100 suppliers
$42.00/100mg
Yield:20348-16-7 99%
Reaction Conditions:
palladium on activated carbon in ethyl acetate;
Steps:
56.a a
a 2-Amino-6-methyl-pyridin-3-ol A mixture of 6-methyl-2-nitro-pyridin-3-ol (18.5 g, 0.120 mmol), 10% palladium on activated carbon (1.35 g), and ethyl acetate (240 mL) was hydrogenated for 3 days. The mixture was filtered through Celite and washed with methanol/ethylacetate (5%). The filtrate and washing were combined and concentrated to give the title compound (14.7 g, 99% yield) as a pale brown solid. 1H NMR (DMSO-d6) δ9.19 (bs, 1H), 6.73 (d, 1H, J=7.6 Hz), 6.12 (d, 1H, J=7.6 Hz), 5.36 (bs, 2H), 2.15 (s, 3H).
References:
US2002/169200,2002,A1
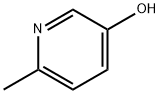
1121-78-4
286 suppliers
$6.00/5g

20348-16-7
100 suppliers
$42.00/100mg