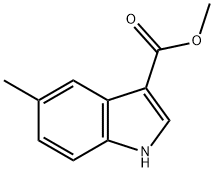
5-METHYLINDOLE-3-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:5-METHYLINDOLE-3-CARBOXYLIC ACID METHYL ESTER
- CAS Number:227960-12-5
- Molecular formula:C11H11NO2
- Molecular Weight:189.21

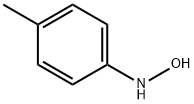
67-56-1
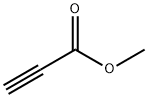
10242-02-1

227960-12-5
GENERAL PROCEDURE: Following the above procedure, the crude reaction mixture was diluted with methanol (MeOH) and cooled to 0 °C. Sulfoxide chloride (SOCl2, 36 μL, 0.5 mmol) was then slowly added and the reaction was stirred at room temperature for 3 hours. Upon completion of the reaction, additional methanol (MeOH) was added and the reaction mixture was heated to reflux for 6 hours. At the end of the reaction, the reaction was quenched with deionized water (H2O) and extracted with ethyl acetate (EtOAc, 3 times). All organic layers were combined and dried with anhydrous magnesium sulfate (MgSO4) and subsequently concentrated under reduced pressure. Finally, the crude product was purified by preparative thin layer chromatography (TLC, unfolding reagent ratio petroleum ether: ethyl acetate = 7:3) to afford the target compounds, methyl 5-methylindole-3-carboxylate (5a-b).

67-56-1
790 suppliers
$7.29/5ml-f

10242-02-1
84 suppliers
$8.00/100mg

227960-12-5
84 suppliers
$25.00/100mg
Yield: 90%
Reaction Conditions:
with thionyl chloride at 0 - 20; for 9 h;Reflux;
Steps:
For the derivativation of indole-3-carboxylic acids 2b and 2k
General procedure: Following the above procedure, the crude reaction mixture was diluted with MeOH and cooled to 0°C. Then SOCl2 (36 μL, 0.5 mmol) was added and stirred for 3 h at room temperature. Additional MeOH was added and the reaction mixture was heated to reflux for 6 h. The resulting mixture was quenched with H2O and extracted with EtOAc (3x). The combined organic layers were dried over MgSO4 and concentrated under reduced pressure. The resulting crude residue was purified by preparative TLC (hexane:EtOAc = 7:3) to afford the desired products 5a-b.
References:
Yoo, Woo-Jin;Nguyen, Thanh V. Q.;Guiteras Capdevila, Montse;Kobayashi, Shu [Heterocycles,2015,vol. 90,# 2,p. 1196 - 1204]

124-41-4
730 suppliers
$12.00/25g

227960-12-5
84 suppliers
$25.00/100mg
614-96-0
324 suppliers
$19.00/1g

227960-12-5
84 suppliers
$25.00/100mg

10242-02-1
84 suppliers
$8.00/100mg

227960-12-5
84 suppliers
$25.00/100mg